Clinical trials are at the heart of developing and implementing new therapies, especially in oncology. However, a large proportion of these trials fail to end informatively, meaning they don’t substantively guide clinical, policy, or research decisions.
We are working with the definition of “informativeness” proposed by Zarin et al in Harms From Uninformative Clinical Trials, JAMA 2019.[1]
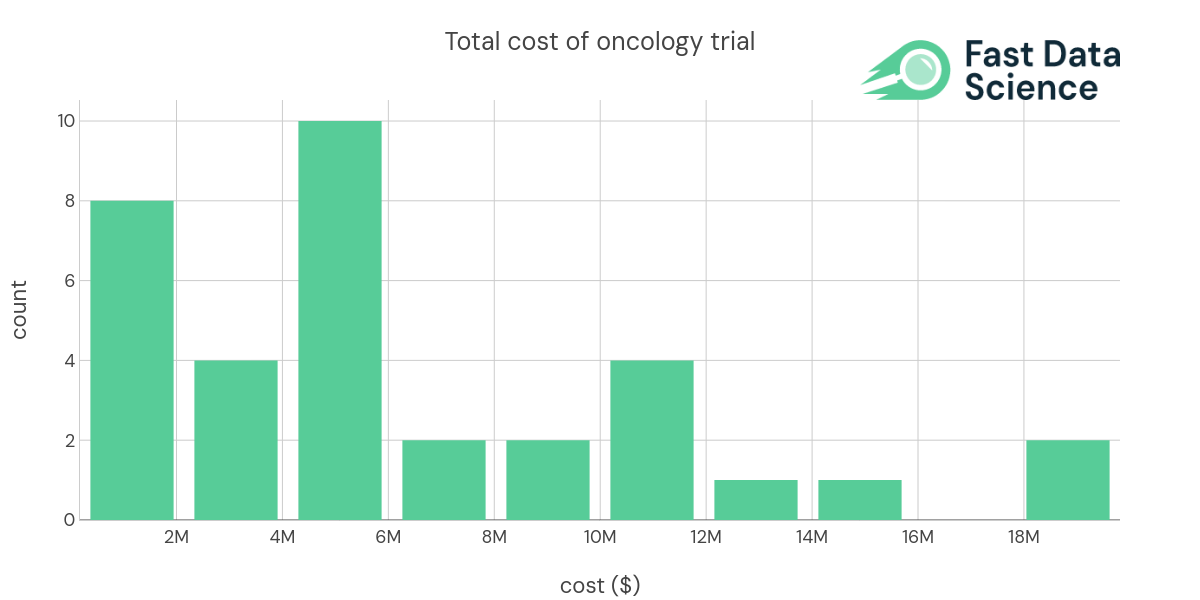
Understanding the potential risk factors involved in non-informative trials is crucial for improving the trial design as well as saving cost and time. Conducting an oncology clinical trial is expensive. Fast Data Science found the median per-patient cost to be $91370 for 35 cancer trials with public data.
Fast Data Science’s Clinical Trial Risk Tool comes into play here. Leveraging the power of machine learning, this tool can predict the risk of a trial ending uninformatively from the protocol text, thereby providing early insight into potential issues. You can read more about how the Clinical Trial Risk Tool quantifies the risk of a trial ending without delivering informative results in our article in Clinical Leader.
The tool harnesses machine learning techniques to analyze written trial protocols and predict the potential informativeness of the trial. It considers numerous factors that may affect the trial’s informativeness, such as the number of participants, length of trial, number of uncontrolled variables, ability to control exposure, etc.
| indication (longer) | Technology | NCT ID | Enrollment | Trial Phase | Total Cost | Per Patient Cost ($PP) |
|---|---|---|---|---|---|---|
| Advanced Myeloid Malignancy | biologic drug | 30 | Phase 1 | 328000 | 10933.3 | |
| Blood Cancer | biologic drug | NCT03483324 | 9 | Phase 1 | 5000000 | 555556 |
| Blood Cancer | biologic drug | NCT03925935 | 24 | Phase 1 | 6192579 | 258024 |
| B cell cancers, Leukemia | biologic drug | NCT03088878 | 156 | Phase 1/2 | 18292674 | 117261 |
| Blood Cancer | biologic drug | NCT02222688 | 26 | Phase 1 | 4179598 | 160754 |
| Colon Cancer | biologic drug | NCT02953782 | 112 | Phase 1/2 | 10234048 | 91375.4 |
| Leukemia, Acute Myeloid (AML) | biologic drug | NCT03248479 | 96 | Phase 1 | 5000000 | 52083.3 |
| Blood Cancer, Solid Tumors | biologic drug | NCT02216409 | 88 | Phase 1 | 6505568 | 73926.9 |
| Breast Cancer | biologic drug | NCT00781612 | 720 | Phase 3 | - | 104186 |
| Stage IV Melanoma | cell therapy | NCT00438984 | 11 | Phase 1 | 936164 | 85105.8 |
| Stage IV Breast Cancer | cell therapy | NCT00791037 | 23 | Phase 1/2 | 2236359 | 97233 |
| Non-Small Cell Lung Cancer | cell therapy | NCT00850785 | 6 | Phase 1 | 653850 | 108975 |
| Brain Cancer | cell therapy | NCT02546102 | 414 | Phase 3 | 5391016 | 13021.8 |
| Leukemia, Acute Myeloid (AML) | cell therapy | NCT03301597 | 146 | Phase 2 | 4310000 | 29520.5 |
| Melanoma | cell therapy | NCT01875653 | 4 | Phase 3 | 3000000 | 750000 |
| Blood Cancer, Bone Marrow Transplant and Viral Infection | cell therapy | NCT03475212 | 60 | Phase 1/2 | 4825587 | 80426.4 |
| Brain Cancer | cell therapy | NCT02208362 | 92 | Phase 1 | 12753854 | 138629 |
| Brain Cancer, Breast Cancer | cell therapy | NCT03696030 | 39 | Phase 1 | 9015149 | 231158 |
| Multiple Myeloma | cell therapy | NCT03288493 | 180 | Phase 1 | 19813407 | 110074 |
| B cell cancers, Leukemia | cell therapy | NCT03233854 | 57 | Phase 1 | 11034982 | 193596 |
| Lung Cancer | cell therapy | NCT03546361 | 36 | Phase 1 | 11815315 | 328203 |
| Melanoma, Skin cancer | cell therapy | NCT03240861 | 12 | Phase 1 | 14144221 | 1.17869e+06 |
| Sarcoma | cell therapy | NCT03240861 | 12 | Phase 1 | 4693839 | 391153 |
| HIV-related Lymphoma, HIV/AIDS | gene therapy | NCT02797470 | 18 | Phase 1/2 | 8414265 | 467459 |
| Prostate cancer | small molecule drug | 232 | Phase 2/3 | 2969523 | 12799.7 | |
| Acute Myeloid Leukemia | small molecule drug | 60 | Phase 1/2 | 1166746 | 19445.8 | |
| Non-Small Cell Lung Cancer | small molecule drug | 140 | Phase 2 | 5852288 | 41802.1 | |
| Solid Tumors | small molecule drug | NCT01954316 | 48 | Phase 1 | 5683693 | 118410 |
Machine learning algorithms trained on historical protocols can develop predictive models on whether a given trial is likely to be informative or not. The more data the algorithm has, the better its predictive accuracy becomes.
Identifying at-risk trial candidates early on using a predictive model allows for valuable time and resources to be redirected towards more promising trials. It can help in focusing efforts on the trials most likely to yield clinically relevant information.
Moreover, the insights generated by the risk tool can also guide improvements in the design and implementation of future trials. By identifying common factors associated with trial un-informativeness, researchers can better orchestrate their strategies to minimize these risk factors.
In conclusion, Fast Data Science’s Clinical Trial Risk Tool, with its ability to predict the risk of failure of oncology clinical trails from protocol text, represents a promising method to optimize the planning and execution of these important studies. Using machine learning technology, it can potentially save significant resources while increasing the quality and potential impact of clinical trials in oncology.