
Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan
Clinical trials are essential for advancing medical science, yet they are inherently complex and involve a wide range of risks. As a result, effective risk management in clinical trials is crucial to ensuring their successful completion.
Among the various approaches to managing these risks, clinical trials Key Risk Indicators (KRIs) have become essential tools. KRIs are precise, measurable metrics that serve as early alerts for potential risk exposures in clinical studies. They provide objective data points that help identify when certain aspects of a trial are trending toward risk, allowing for timely corrective actions.
It’s important to distinguish KRIs from Key Performance Indicators (KPIs); while KPIs measure achievement of predefined goals—such as meeting patient recruitment targets or completing scheduled monitoring visits—KRIs focus exclusively on identifying deviations or risks that could negatively impact study outcomes.
A well-constructed clinical trial protocol is fundamental to research success, yet risks embedded in the study design can jeopardize outcomes if left unaddressed. Essential elements to monitor include the complexity of the protocol, feasibility of endpoints, accuracy of sample size and power calculations, proper execution of randomization and blinding, and study duration. Early evaluation of these design risks enables sponsors to proactively address potential challenges before they adversely affect trial results.
One way to assess your protocol for design risks is to drag and drop it into the free Clinical Trial Risk Tool. You will see a list of recommendations around the statistical analysis plan, sample size, etc, like in the screenshot below.
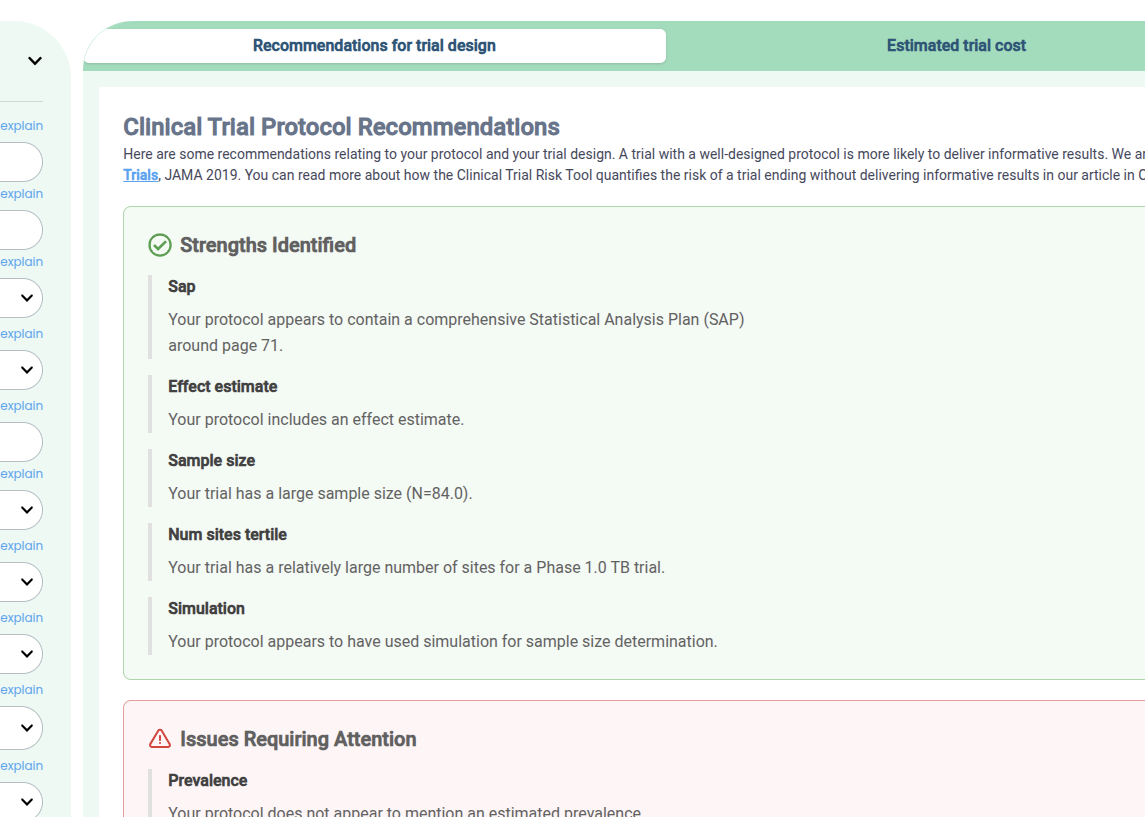
Recommendations produced by the Clinical Trial Risk Tool for a protocol
Check your trial design
This indicator measures the pace and effectiveness of enrolling participants against the planned recruitment timeline. Timely recruitment is critical; delays can cause costly setbacks and compromise the statistical power needed for conclusive results. Patient recruitment rate is especially important in trials involving rare diseases, where patient availability is limited. You can read more in Allucent’s enlightening post on Rare Disease Clinical Trials: Challenges, Strategies, and Solutions for Success. Tracking recruitment performance across sites helps identify bottlenecks or operational hurdles early, allowing sponsors to adjust strategies by adding new sites or enhancing recruitment efforts.
Protocol deviations occur when study sites or investigators fail to follow the approved clinical protocol. There is (FDA Draft Guidance)[https://www.fda.gov/media/184745/download] offering recommendations to assist sponsors, investigators, and institutional review boards (IRBs) in defining, identifying, and reporting protocol deviations during clinical trials. A rise in protocol deviations may indicate training deficiencies, unclear protocol language, or inadequate site oversight. Monitoring this KRI closely allows for timely corrective measures including retraining, clarifying protocols, or increasing monitoring activities to safeguard trial integrity.
This KRI reflects the frequency of data clarifications or corrections requested from study sites. Elevated query rates can point to data quality issues like entry errors, misunderstandings of case report forms, or systemic problems in data capture. High volumes of data queries can delay key milestones, including database lock, and jeopardize the reliability of trial findings. Monitoring data query rates helps identify underperforming sites or problematic processes, enabling targeted corrective actions such as additional training or system improvements.
Patient dropout rate tracks the percentage of participants who withdraw from the study before completion. Elevated dropout rates threaten study validity by reducing sample size and potentially introducing bias, especially if dropouts relate to adverse treatment effects. Monitoring this indicator assists in uncovering causes such as adverse events, logistical challenges, or lack of patient engagement. Based on dropout trends, trial teams can implement retention strategies like enhanced communication, patient support programs, or improved follow-up protocols to maintain participant continuity.
The occurrence and pattern of serious adverse events provide a direct measure of patient safety and treatment risk. Unexpected increases in SAEs require immediate investigation and may prompt protocol amendments, increased monitoring, or even temporary study suspension. Continuous tracking of SAEs also ensures compliance with regulatory reporting requirements and reinforces participant protection.
This KRI monitors how consistently site visits occur as scheduled. Regular monitoring visits are crucial for ensuring protocol adherence, validating data accuracy, and protecting participant safety. Missed or delayed visits can allow risks to go unnoticed and escalate. By tracking visit frequency, sponsors can focus oversight on high-risk sites and optimize resource use, particularly within a risk-based monitoring framework.

The adoption of KRIs in clinical trials has shown a significant and consistent rise over recent years. A detailed survey examining 5,987 ongoing clinical trials in 2020 revealed that nearly 77% of these studies incorporated at least one component of Risk-Based Monitoring (RBM), with KRIs being a prominent feature [2]. This growing trend highlights the increasing recognition of KRIs as essential tools for proactive risk management in clinical trials.
Looking ahead, the future of KRIs in clinical research is intimately tied to advancements in technology [3]. Artificial intelligence (AI) and machine learning stand at the forefront of this transformation, offering the ability to analyze vast and complex datasets far beyond human capacity. These technologies enable more accurate prediction of emerging risks, allowing for dynamic adjustment of KRI definitions and thresholds. This adaptive approach leads to earlier detection of potential issues and more focused interventions, significantly enhancing the efficiency and effectiveness of clinical trial monitoring.
In addition to theoretical promise, several AI-powered tools have already been developed to anticipate risks in clinical studies. For instance, the Clinical Trial Risk Tool utilizes AI to identify risks associated with trial design and the possibility that a trial may conclude without producing meaningful results. The tool also estimates the financial cost associated with the trial. In addition, it also offer the practical recommendations to mitigate the identified risks.
KRIs have become indispensable tools in managing the inherent complexities and risks of clinical trials. By providing early, measurable signals of potential issues, KRIs empower trial teams to safeguard patient safety, maintain data integrity, and ensure regulatory compliance. Their strategic use helps prevent costly disruptions and optimizes resource allocation, ultimately enhancing overall trial performance and success.
de Viron, S., et al., Does central monitoring lead to higher quality? an analysis of key risk indicator outcomes. Therapeutic Innovation & Regulatory Science, 2023. 57 (2): p. 295-303.
Stansbury, N., et al., Risk-Based Monitoring in Clinical Trials: Increased Adoption Throughout 2020. Therapeutic Innovation & Regulatory Science, 2022. 56 (3): p. 415-422.
Kolluri, S., et al., Machine learning and artificial intelligence in pharmaceutical research and development: a review. The AAPS journal, 2022. 24: p. 1-10.

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Upload your clinical trial protocol and create a cost benchmark with AI Protocol to cost benchmark The Clinical Trial Risk Tool uses AI and Natural Language Processing (NLP) to estimate the cost of a trial using the information contained in the clinical trial protocol.

You can download a white paper about clinical trial cost benchmarking here Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.