In recent years, the costs of clinical trials have soared, with a notable increase observed in oncology trials. According to the Pharmaceutical Research and Manufacturers of America (PhRMA), oncology trials accounted for more than 40% of industry-sponsored trials with an average per-patient expense of $59,500. More recent estimates by Fast Data Science place the median per-patient cost for cancer trials at $91,370. Such high costs underscore the necessity for trials to yield meaningful and informative results.
But what do we mean by ‘informativeness’? Essentially, the ‘informativeness’ of a trial refers to its ability to guide medical, policy, or research decisions. In other words, the trial does not merely exist to ascertain facts but also to influence action beneficial to patients.
A recent study by Hutchinson et al (2022) provided new insight into the proportion of randomized controlled trials that indeed prove informative. Among 125 clinical trials in three disease areas (ischemic heart disease, diabetes mellitus, and lung cancer), they found that only 26.4% met the conditions for informativeness.
Given the high costs and the relatively low informativeness rate, there is a pressing need for early prediction tools that can estimate the informativeness of a given trial. This is precisely where machine learning comes into play, and Fast Data Science’s Clinical Trial Risk Tool offers a promising solution.
| indication (longer) | Technology | CT.gov URL | Enrollment | Trial Phase | Total Cost | Per Patient Cost ($PP) |
|---|---|---|---|---|---|---|
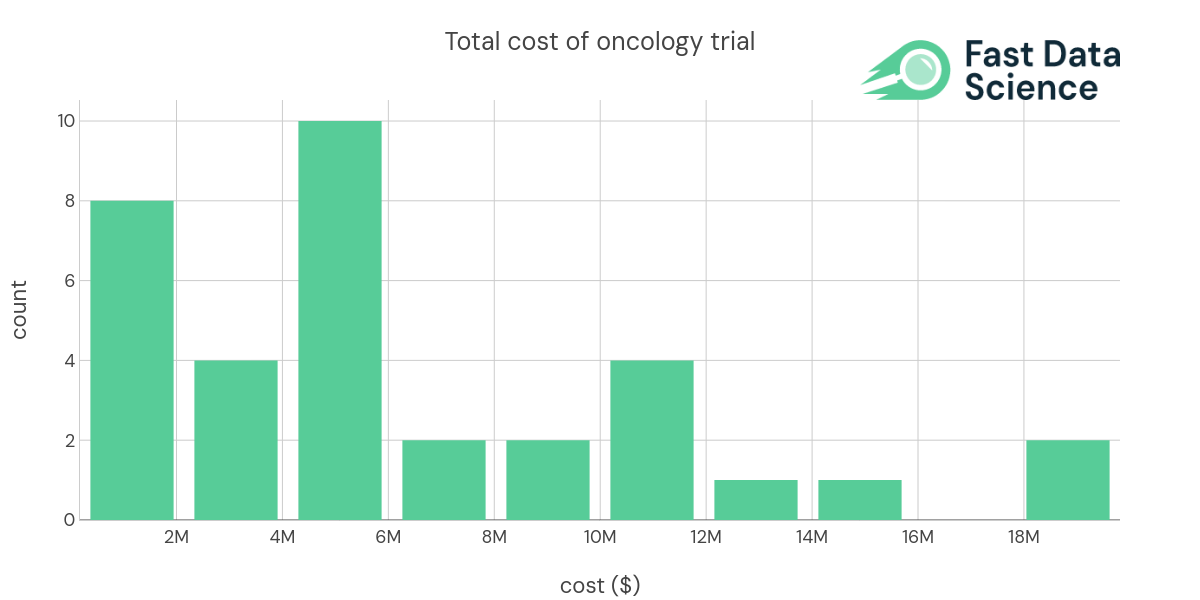
| Advanced Myeloid Malignancy | biologic drug | 30 | Phase 1 | 328000 | 10933.3 | |
| Blood Cancer | biologic drug | NCT03483324 | 9 | Phase 1 | 5000000 | 555556 |
| Blood Cancer | biologic drug | NCT03925935 | 24 | Phase 1 | 6192579 | 258024 |
| B cell cancers, Leukemia | biologic drug | NCT03088878 | 156 | Phase 1/2 | 18292674 | 117261 |
| Blood Cancer | biologic drug | NCT02222688 | 26 | Phase 1 | 4179598 | 160754 |
| Colon Cancer | biologic drug | NCT02953782 | 112 | Phase 1/2 | 10234048 | 91375.4 |
| Leukemia, Acute Myeloid (AML) | biologic drug | NCT03248479 | 96 | Phase 1 | 5000000 | 52083.3 |
| Blood Cancer, Solid Tumors | biologic drug | NCT02216409 | 88 | Phase 1 | 6505568 | 73926.9 |
| Breast Cancer | biologic drug | NCT00781612&draw=2&rank=1 | 720 | Phase 3 | - | 104186 |
| Stage IV Melanoma | cell therapy | NCT00438984 | 11 | Phase 1 | 936164 | 85105.8 |
| Stage IV Breast Cancer | cell therapy | NCT00791037 | 23 | Phase 1/2 | 2236359 | 97233 |
| Non-Small Cell Lung Cancer | cell therapy | NCT00850785 | 6 | Phase 1 | 653850 | 108975 |
| Brain Cancer | cell therapy | NCT02546102 | 414 | Phase 3 | 5391016 | 13021.8 |
| Leukemia, Acute Myeloid (AML) | cell therapy | NCT03301597 | 146 | Phase 2 | 4310000 | 29520.5 |
| Melanoma | cell therapy | NCT01875653 | 4 | Phase 3 | 3000000 | 750000 |
| Blood Cancer, Bone Marrow Transplant and Viral Infection | cell therapy | NCT03475212 | 60 | Phase 1/2 | 4825587 | 80426.4 |
| Brain Cancer | cell therapy | NCT02208362 | 92 | Phase 1 | 12753854 | 138629 |
| Brain Cancer, Breast Cancer | cell therapy | NCT03696030 | 39 | Phase 1 | 9015149 | 231158 |
| Multiple Myeloma | cell therapy | NCT03288493 | 180 | Phase 1 | 19813407 | 110074 |
| B cell cancers, Leukemia | cell therapy | NCT03233854 | 57 | Phase 1 | 11034982 | 193596 |
| Lung Cancer | cell therapy | NCT03546361 | 36 | Phase 1 | 11815315 | 328203 |
| Melanoma, Skin cancer | cell therapy | NCT03240861 | 12 | Phase 1 | 14144221 | 1.17869e+06 |
| Sarcoma | cell therapy | NCT03240861 | 12 | Phase 1 | 4693839 | 391153 |
| HIV-related Lymphoma, HIV/AIDS | gene therapy | NCT02797470 | 18 | Phase 1/2 | 8414265 | 467459 |
| Prostate cancer | small molecule drug | 232 | Phase 2/3 | 2969523 | 12799.7 | |
| Acute Myeloid Leukemia | small molecule drug | 60 | Phase 1/2 | 1166746 | 19445.8 | |
| Non-Small Cell Lung Cancer | small molecule drug | 140 | Phase 2 | 5852288 | 41802.1 | |
| Solid Tumors | small molecule drug | NCT01954316 | 48 | Phase 1 | 5683693 | 118410 |
The tool, which leverages the power of machine learning, analyses protocol text to forecast the informativeness of a trial. This predictive ability is based on several factors likely to impact a trial’s informativeness: the number of participants, the duration of the trial, the number of uncontrolled variables, the ability to control exposure, and completion of a Statistical Analysis Plan (SAP).
So how does it work? The tool essentially learns patterns from previous trial protocols and outcomes, then applies these patterns to future trials, making it capable of extracting value from a large and complicated dataset. It’s a process that would be arduous and likely less accurate if undertaken by humans alone.
In conclusion, while the informativeness of oncology clinical trials is presently not optimal considering the high associated costs, predictive models like the Clinical Trial Risk Tool prove instrumental in ushering an era of more effective, goal-oriented trials. The tool is a testament to the transformative power of machine learning to impact healthcare and potentially improve patient outcomes.