Creating a clinical trial budget is a fiddly and time consuming process. The playbook for running the clinical trial is a document called the protocol. You can find examples of protocols here. The protocol states how many participants will take part in the trial and also what visits and procedures will take place.
A clinical trial manager must read the protocol and look for all pieces of information in the protocol that is relevant to the budget, in particular the Schedule of Events (also called Schedule of Assessments or Schedule of Activities), which is a table or series of tables which indicate which procedures and assessments will take place on which the visits. By looking at the Schedule of Events, you can see immediately, for example, how many blood tests each patient will undergo.
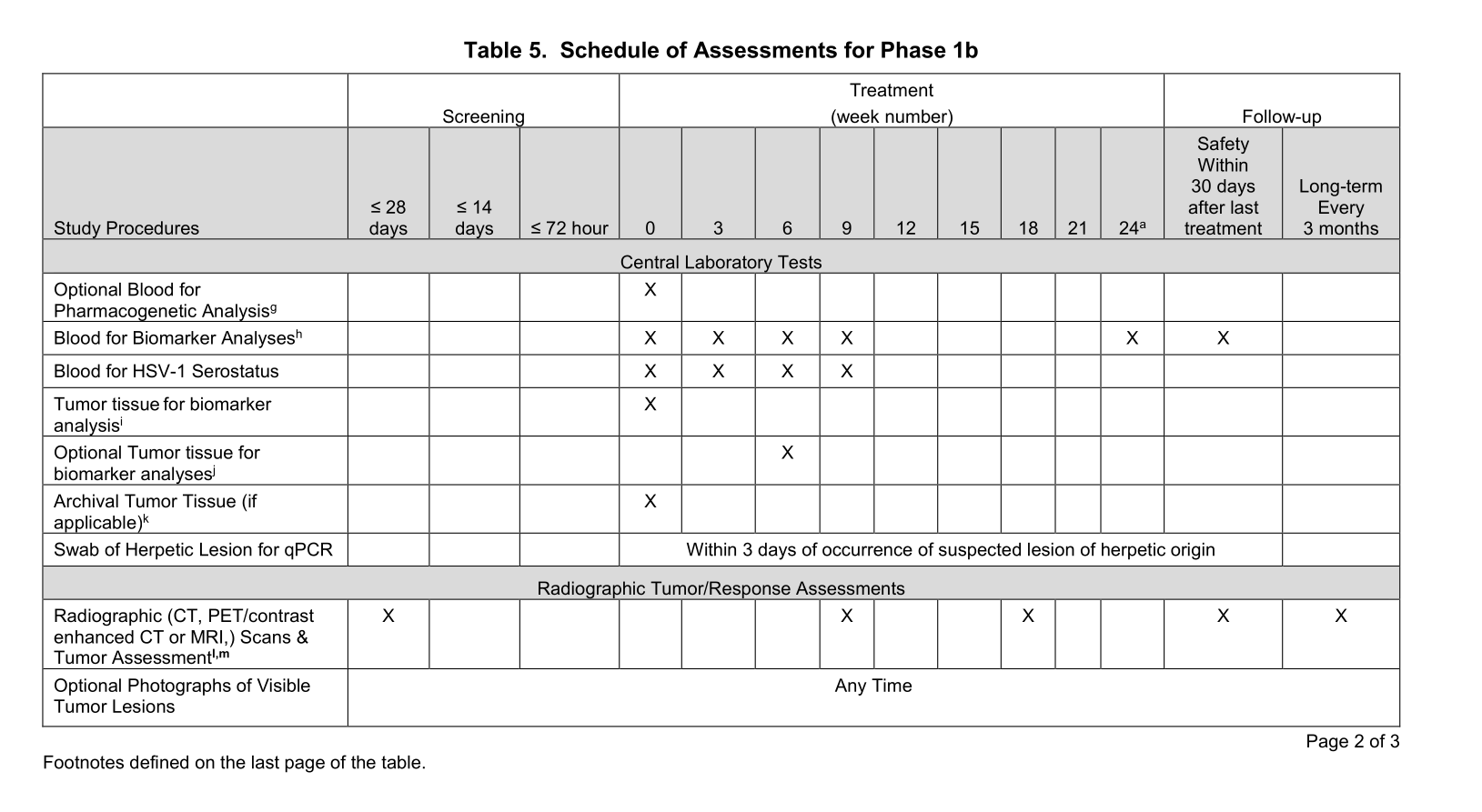
Above: a schedule of events. Source: NCT02626000
When a trial manager is creating a budget they will find the procedures in the Schedule of Events and also look through the footnotes to work out if there are any extra bits of information not contained in the table.
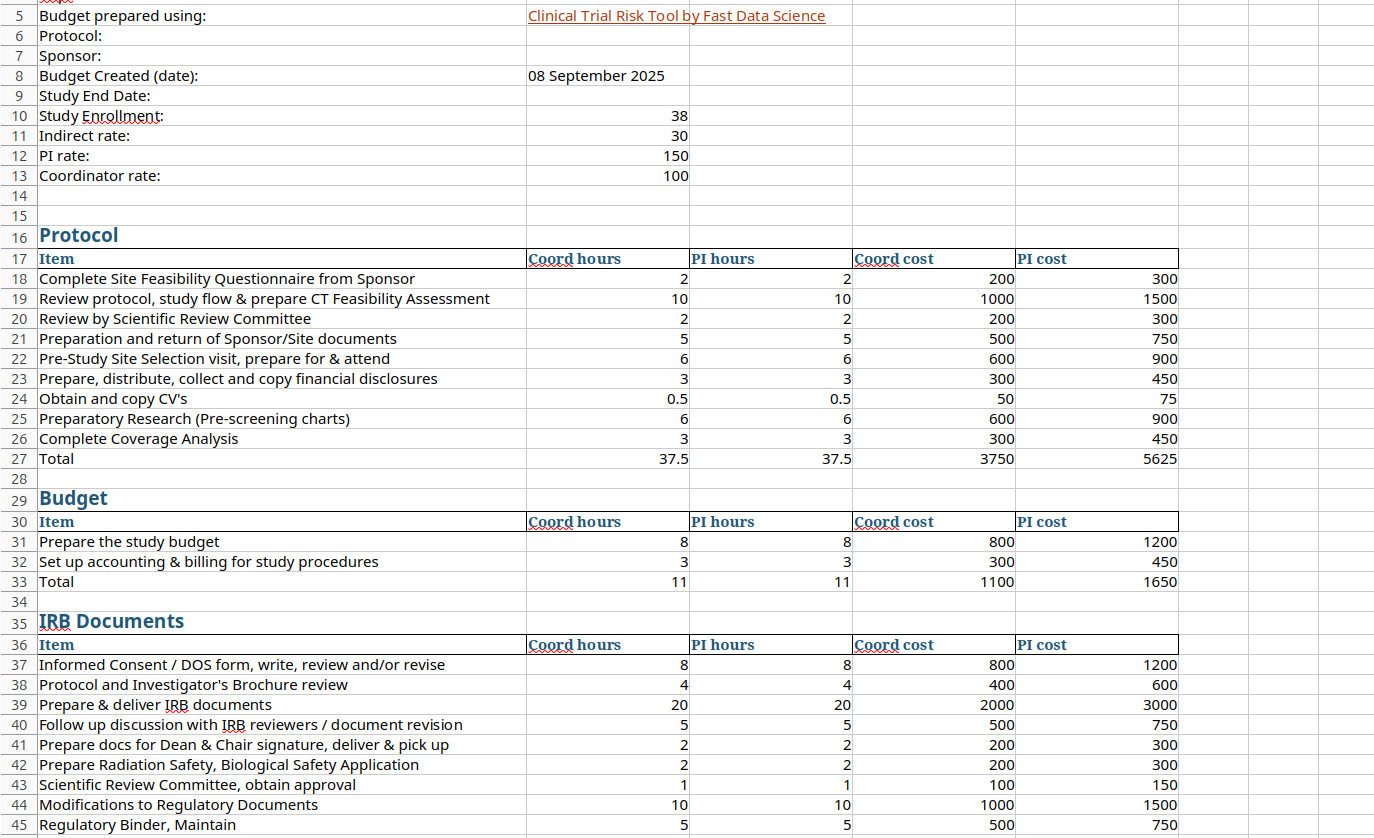
They then transform the schedule of events into a spreadsheet format. The budget spreadsheet contains a tab detailing every blood test, every MRI scan, and every moment that a patient is weighed or a patient has to fill out a consent form. The process of transferring this information from the protocol into the schedule of events involves a lot of copying and pasting, and a lot of translating procedure names from plain English into look up codes such as ICD codes, and looking them up in tables of costs. The end result of this process is a budget in Excel format, constructed from the bottom up.
The process of creating an itemised budget from the protocol is slow and error prone, slightly subjective, and involves cross referencing information from multiple sources, such as where CROs or investigational sites have provided cost information. There may be information about costs inside the contract issued by the trial sponsor.
The clinical trial manager may need to do some research to find out how much certain procedures cost and then perhaps compare different providers to find a competitive rate for each of the activities.
All of this results in a rather complicated spreadsheet consisting of several tabs which can be subject to change and iteration, for example, where the sponsor or CRO switches sites, or where the protocol is amended
The Clinical Trial Risk Tool was originally developed to assess the risk of clinical trial protocols, in particular the risk of a trial failing to deliver informative results.
A number of users then asked Fast Data Science if we could extend the tool to create an itemised site budget. We have implemented this with a mixture of traditional Natural Language Processing and cutting-edge generative AI.
Check your trial budget
We first of all use the main part of the Clinical Trial Risk Tool to pick out key numbers relevant to the cost. The most important number is the sample size - it’s very important that we get this right. Read more about how the Clinical Trial Risk Tool calculates sample sizes.
The protocol itself is a huge document and it is not desirable to send the entire PDF to a generative AI model.
It contains too many pages and too many tokens to be handled by gen AI
When Gen AI models are sent too much information, they tend to hallucinate
Fortunately, traditional (pre-gen-AI) language models such as Naive Bayes and Convolutional Neural Network classifiers are light weight and can easily identify the pages containing the Schedule of Events and its footnotes. We use these to find the informative parts of the protocol. For example, the statistical analysis section is not very relevant for budget generation and we don’t need it when we are looking for the Schedule of Events.
Having found the relevant pages, we slice up the protocol and create a smaller version of it which we can send to a generative AI model.
We use this model to convert the table to CSV and ultimately an Excel format. We upload the PDF and use an engineered prompt to retrieve only the Schedule of Events but not the irrelevant information in the protocol.
Why use generative AI to read the Schedule of Events?
We have found that the Schedule of Events can be very varied in format, from simple tables, to tables using cell colours or shading to indicate an event taking place, to a plain image embedded in the PDF. For that reason, deterministic or programmatic approaches haven’t really worked, and generative AI models have been the only solution we have found to reading this table from the PDF.
The Clinical Trial Risk Tool comes with its own cost databases for how much procedures cost in different locations and currencies. The user can also upload their own cost databases.
This innovation allows clinical trial professionals to concentrate on what they are good at, namely running clinical trials. They can leave the time consuming task of creating complicated multitabbed Excels to a computer and AI system.
The Clinical Trial Risk Tool’s budget add-on will save hours of your time as a clinical research professional.
We have received feedback that the Clinical Trial Risk Tool’s risk assessment cuts the average protocol review time from 8 hours to one hour. We hope that similar cost-cutting and time savings will be achieved with the budget generation functionality.
Sign up at https://clinicaltrialrisk.org/ to try the Clinical Trial Risk Tool.
Contact us to have access to the budget generation add-on.
A few years ago, the idea of using AI to generate a clinical trial budget would have been science fiction. Even today, with the advent of generative AI, organisations in certain Industries have been reticent to adopt AI for business critical processes, due to concerns about the reliability of generative AI. Because the Clinical Trial Risk Tool uses generative AI only where strictly necessary, and combines it with traditional natural language processing and a bespoke cost database, and the budget generation is based on knowledge provided by clinical research professionals who are doing this in their day-to-day jobs, the tool is likely to generate a reliable budget you can trust, which needs minimal edits after it has been generated.

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Upload your clinical trial protocol and create a cost benchmark with AI Protocol to cost benchmark The Clinical Trial Risk Tool uses AI and Natural Language Processing (NLP) to estimate the cost of a trial using the information contained in the clinical trial protocol.

You can download a white paper about clinical trial cost benchmarking here Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.