Clinical trials are the lifeblood of modern healthcare innovation. They’re how we test new treatments, evaluate current standards of care, and identify areas for improvement. However, as a recent study by Markey et al indicates, these trials are becoming more complex over time, particularly in Oncology, leading to lengthier trials and lower success rates. This article explores these trends and introduces an innovative solution from Fast Data Science – a Clinical Trial Risk Tool that uses machine learning to predict the complexity of a clinical trial from the protocol text.
Based on an analysis of over 16,000 clinical trials, the study found a significant increase in trial complexity across multiple therapeutic areas. Oncology stood out as the area with the most complex trials, with indications such as prostate, colorectal, breast and lung cancer contributing heavily. Though this trend appeared to level off slightly in 2020, presumably due to the impacts of COVID-19 on trial planning, the overall trajectory shows an increasingly complex landscape.
So, how can we navigate this complexity? This is where Fast Data Science’s Clinical Trial Risk Tool comes in. This innovative tool uses a machine learning algorithm to assess key features of a trial, such as the number of endpoints, inclusion-exclusion criteria, and more, from the protocol text. It then predicts the trial’s complexity by assigning it a Trial Complexity Score, a new metric introduced by Markey et al.
| indication (longer) | Technology | CT.gov URL | Enrollment | Trial Phase | Total Cost | Per Patient Cost ($PP) |
|---|---|---|---|---|---|---|
| Advanced Myeloid Malignancy | biologic drug | 30 | Phase 1 | 328000 | 10933.3 | |
| Blood Cancer | biologic drug | NCT03483324 | 9 | Phase 1 | 5000000 | 555556 |
| Blood Cancer | biologic drug | NCT03925935 | 24 | Phase 1 | 6192579 | 258024 |
| B cell cancers, Leukemia | biologic drug | NCT03088878 | 156 | Phase 1/2 | 18292674 | 117261 |
| Blood Cancer | biologic drug | NCT02222688 | 26 | Phase 1 | 4179598 | 160754 |
| Colon Cancer | biologic drug | NCT02953782 | 112 | Phase 1/2 | 10234048 | 91375.4 |
| Leukemia, Acute Myeloid (AML) | biologic drug | NCT03248479 | 96 | Phase 1 | 5000000 | 52083.3 |
| Blood Cancer, Solid Tumors | biologic drug | NCT02216409 | 88 | Phase 1 | 6505568 | 73926.9 |
| Breast Cancer | biologic drug | NCT00781612&draw=2&rank=1 | 720 | Phase 3 | - | 104186 |
| Stage IV Melanoma | cell therapy | NCT00438984 | 11 | Phase 1 | 936164 | 85105.8 |
| Stage IV Breast Cancer | cell therapy | NCT00791037 | 23 | Phase 1/2 | 2236359 | 97233 |
| Non-Small Cell Lung Cancer | cell therapy | NCT00850785 | 6 | Phase 1 | 653850 | 108975 |
| Brain Cancer | cell therapy | NCT02546102 | 414 | Phase 3 | 5391016 | 13021.8 |
| Leukemia, Acute Myeloid (AML) | cell therapy | NCT03301597 | 146 | Phase 2 | 4310000 | 29520.5 |
| Melanoma | cell therapy | NCT01875653 | 4 | Phase 3 | 3000000 | 750000 |
| Blood Cancer, Bone Marrow Transplant and Viral Infection | cell therapy | NCT03475212 | 60 | Phase 1/2 | 4825587 | 80426.4 |
| Brain Cancer | cell therapy | NCT02208362 | 92 | Phase 1 | 12753854 | 138629 |
| Brain Cancer, Breast Cancer | cell therapy | NCT03696030 | 39 | Phase 1 | 9015149 | 231158 |
| Multiple Myeloma | cell therapy | NCT03288493 | 180 | Phase 1 | 19813407 | 110074 |
| B cell cancers, Leukemia | cell therapy | NCT03233854 | 57 | Phase 1 | 11034982 | 193596 |
| Lung Cancer | cell therapy | NCT03546361 | 36 | Phase 1 | 11815315 | 328203 |
| Melanoma, Skin cancer | cell therapy | NCT03240861 | 12 | Phase 1 | 14144221 | 1.17869e+06 |
| Sarcoma | cell therapy | NCT03240861 | 12 | Phase 1 | 4693839 | 391153 |
| HIV-related Lymphoma, HIV/AIDS | gene therapy | NCT02797470 | 18 | Phase 1/2 | 8414265 | 467459 |
| Prostate cancer | small molecule drug | 232 | Phase 2/3 | 2969523 | 12799.7 | |
| Acute Myeloid Leukemia | small molecule drug | 60 | Phase 1/2 | 1166746 | 19445.8 | |
| Non-Small Cell Lung Cancer | small molecule drug | 140 | Phase 2 | 5852288 | 41802.1 | |
| Solid Tumors | small molecule drug | NCT01954316 | 48 | Phase 1 | 5683693 | 118410 |
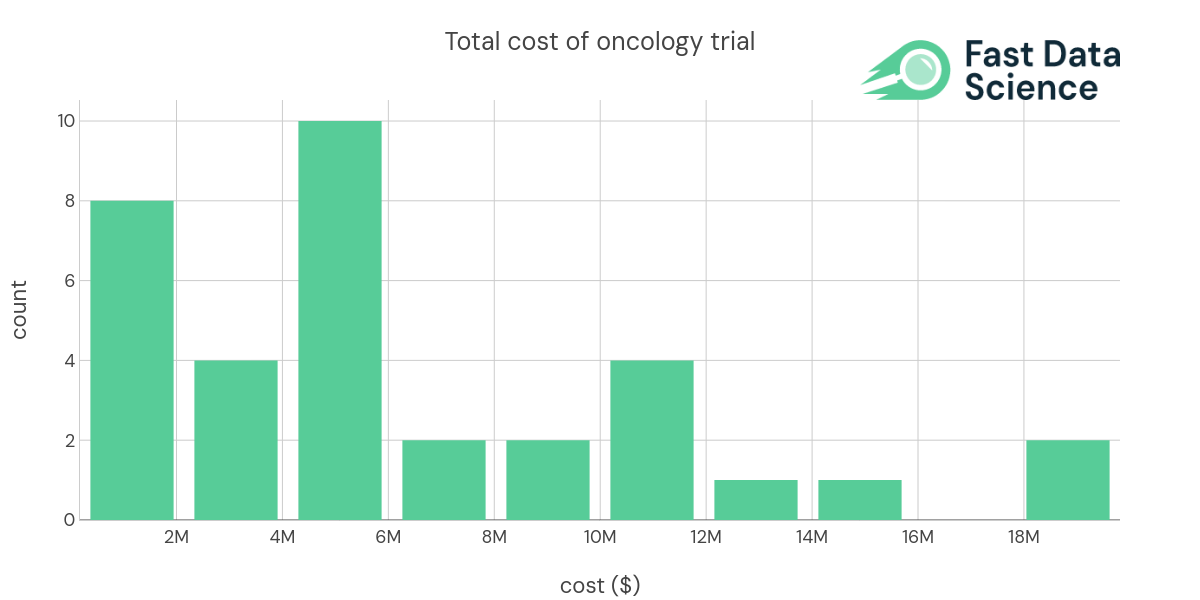
By predicting the complexity of an Oncology trial early in the planning process, researchers can make adjustments to prevent unnecessary complications. It may help in setting realistic trial timelines, budgeting appropriately, choosing the right team and resources, and, ultimately, improving the success rate of these vital trials.
Ultimately, Fast Data Science’s Clinical Trial Risk Tool is a powerful resource to aid in the planning and execution of clinical trials. It arms researchers with essential insights into the likely complexity of a trial, setting them up for success in the challenging world of Oncology clinical trials.
In a world where clinical trial complexity is increasingly becoming the norm, tools like this one from Fast Data Science offer a beacon of hope for a more streamlined and efficient trial process – helping us get life-saving treatments to patients faster, safer, and more efficiently.