The Clinical Trial Risk Tool has been selected as a winner of the Plotly Dash Example Apps Challenge (2023), out of 25 amazing apps submitted by the Dash users community! The trial risk app is built on Plotly Dash, a front end graphical software package.
Thomas Wood from Fast Data Science will be presenting the tool in a webinar on 7 June 2023 which you can book here.
Thank you to all #PlotlyCommunity members who participated in the recent #Dash Example Apps Challenge, and congratulations to the winning submissions!
— Plotly (@plotlygraphs) May 22, 2023
🥇 Clinical Trial Risk Dash App by Thomas Wood
🥈 SARIMA Tuner by Gabriele Albini
🥉 Product Environmental Report Dash App by…
Meanwhile, we have an article published at Wood TA and McNair D. Clinical Trial Risk Tool: software application using natural language processing to identify the risk of trial uninformativeness [version 1; peer review: awaiting peer review]. Gates Open Res 2023, 7:56 (https://doi.org/10.12688/gatesopenres.14416.1).
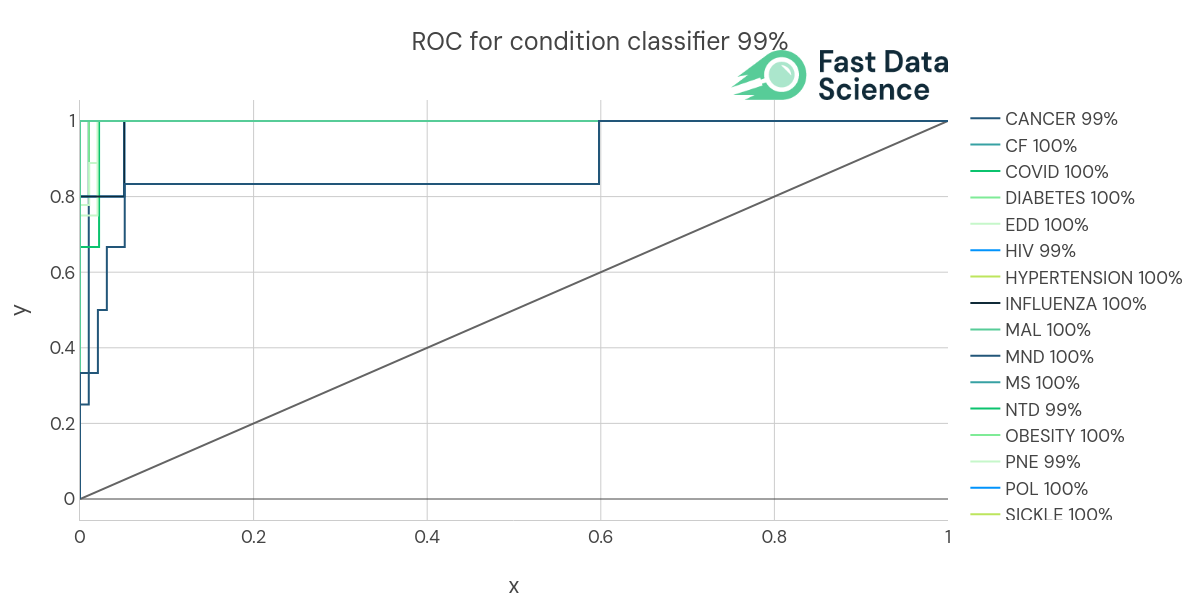
Introduction People have asked us often, how was the Clinical Trial Risk Tool trained? Does it just throw documents into ChatGPT? Or conversely, is it just an expert system, where we have painstakingly crafted keyword matching rules to look for important snippets of information in unstructured documents? Most of the tool is built using machine learning techniques. We either hand-annotated training data, or took training data from public sources. How We Trained the Models inside the Clinical Trial Risk Tool The different models inside the Clinical Trial Risk tool have been trained on real data, mostly taken from clinical trial repositories such as clinicaltrials.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.