
This post originally appeared on Fast Data Science’s blog on LinkedIn.
Clinical trials, the backbone of medical science advancement, often grapple with high costs, complexity, and lengthy timelines. Fast Data Science presents Fast Clinical AI, a game-changing solution that harnesses the power of Natural Language Processing (NLP) and predictive modelling to tackle these challenges head-on.
Fast Clinical AI automates the extraction of critical information from trial protocols, significantly reducing manual efforts. This tool identifies risk factors and predicts costs and enrolment criteria, ensuring efficient trial planning and execution.
By identifying potential risks early in the trial process, Fast Clinical AI allows researchers to take proactive measures, minimising the chances of trial delays or failures. This proactive approach ensures that trials are faster and more reliable.
Fast Clinical AI helps predict and manage the costs associated with clinical trials, ensuring better resource allocation. Reducing manual efforts and streamlining processes significantly reduces the time required to bring new treatments to market.
Explore how Fast Clinical AI can revolutionise your clinical trials. Visit here for more details. Learn more about clinical trial cost modelling with NLP and AI here. Connect with us today to see how we can support your clinical trial efforts. Let’s innovate together!
#ClinicalTrials #AI #NLP #HealthcareInnovation #FastClinicalAI
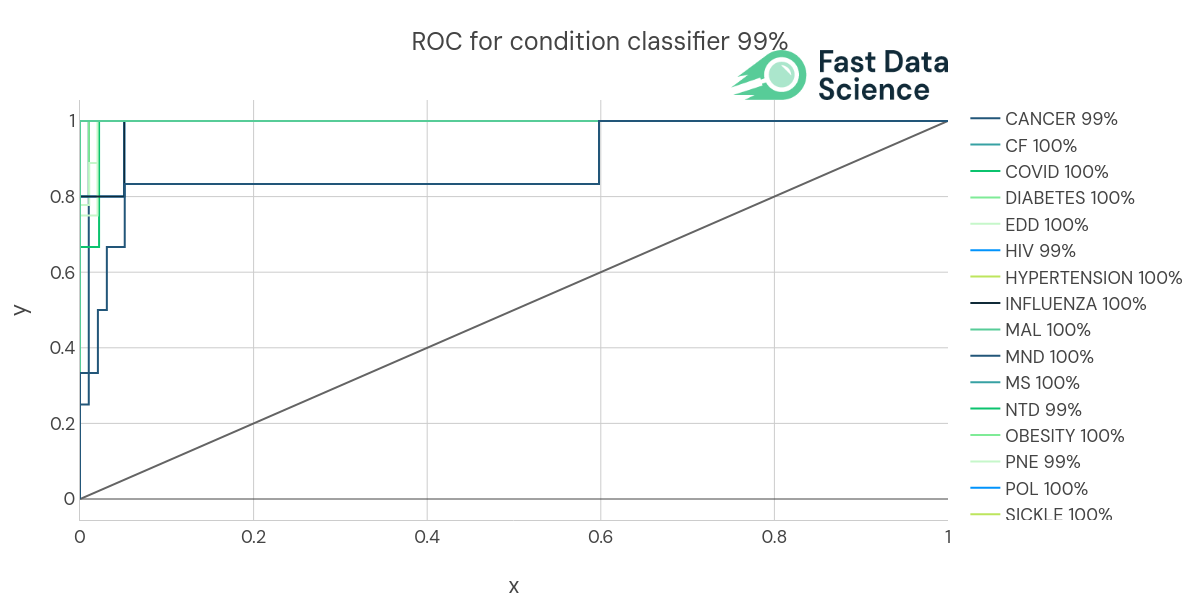
Introduction People have asked us often, how was the Clinical Trial Risk Tool trained? Does it just throw documents into ChatGPT? Or conversely, is it just an expert system, where we have painstakingly crafted keyword matching rules to look for important snippets of information in unstructured documents? Most of the tool is built using machine learning techniques. We either hand-annotated training data, or took training data from public sources. How We Trained the Models inside the Clinical Trial Risk Tool The different models inside the Clinical Trial Risk tool have been trained on real data, mostly taken from clinical trial repositories such as clinicaltrials.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.