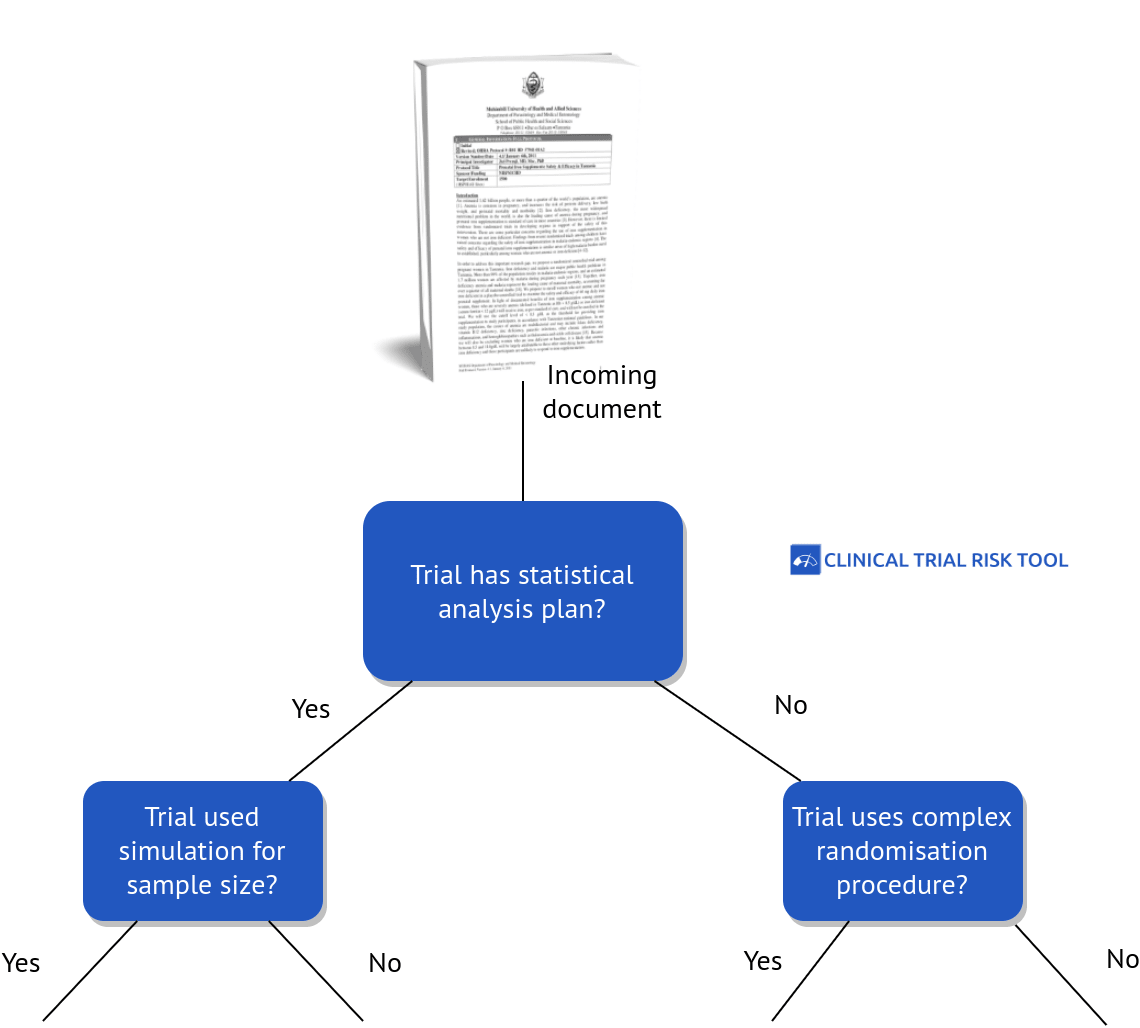
Fast Data Science Ltd (developers of the Clinical Trial Risk Tool) have also worked on a similar natural language processing project for a pharmaceutical company which needed to predict the overall complexity and cost of running a clinical trial. We developed a web-based tool which allowed a non-technical user to drag and drop a PDF file of a clinical trial protocol. The tool converted the PDF to raw text and extracted a number of key properties of the trial, such as the number of subjects, location, number of visits, type of treatment, and so on. These properties were fed into a
risk model which rated the trial as low, medium or high
complexity in three dimensions (subject score, study score, and site score).