
Guest post by Youssef Soliman, medical student at Assiut University and biostatistician
Clinical trial protocols are detailed master-plans of a study – often 100–200 pages long – outlining objectives, design, procedures, eligibility and analysis. Reading them cover-to-cover can be daunting and time-consuming. Yet careful review is essential. Protocols are the “backbone” of good research, ensuring trials are safe for participants and scientifically valid [1]. Fortunately, there are systematic strategies to speed up review and keep it objective. For example, modern AI tools like the Clinical Trial Risk Tool can rapidly parse a protocol’s text, highlighting design issues and estimating cost and risk [2]. These tools flag common risk factors (e.g. missing statistical plans or unusual endpoints) so reviewers can focus their attention. By combining smart software with a structured reading approach, you can cover a protocol far more efficiently.
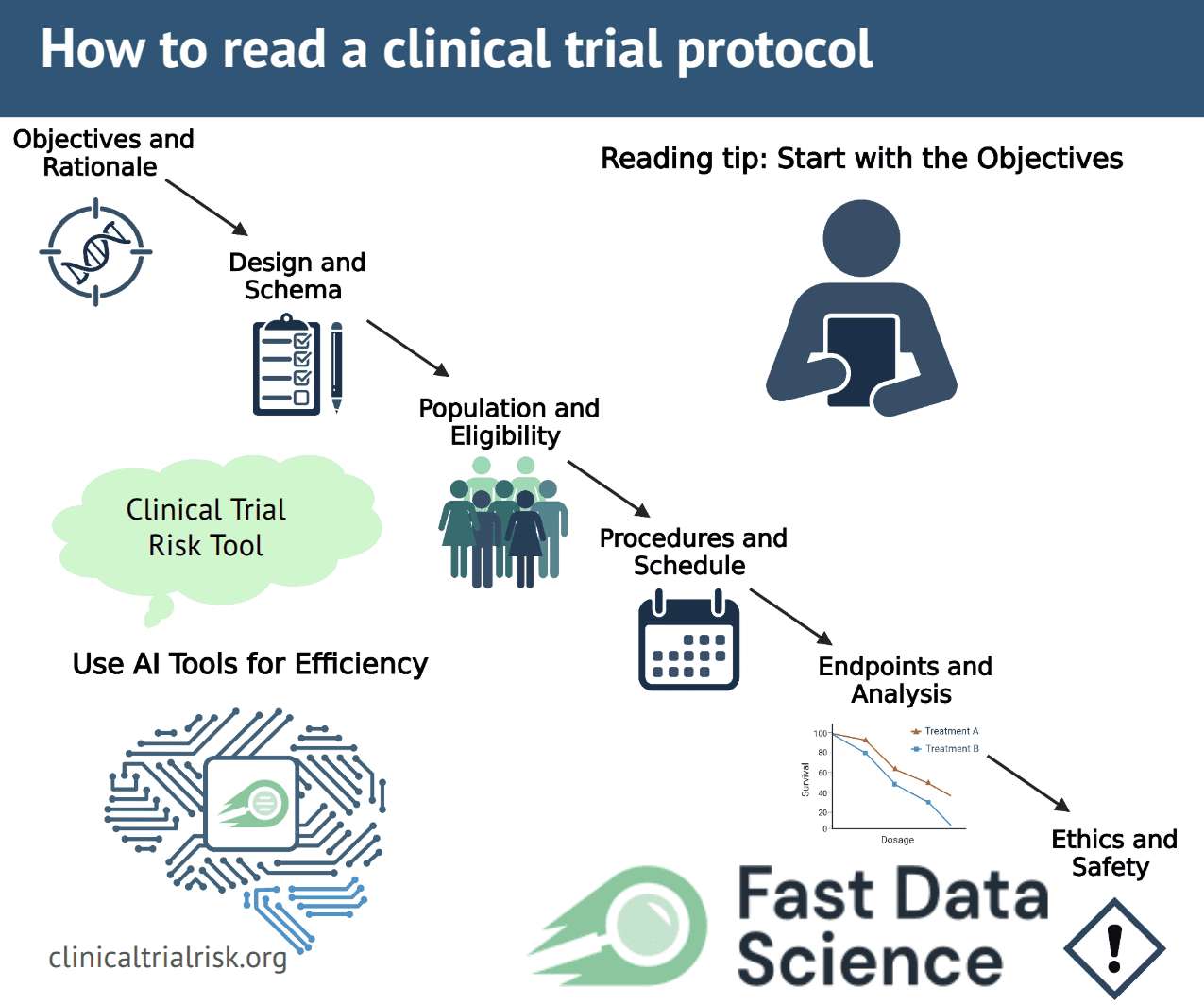
Clinical trial protocols follow standardized guidelines but vary greatly in format. A single protocol may include: background and rationale; specific study objectives; detailed inclusion/exclusion criteria; intervention descriptions; visit schedules; outcome measures; statistical plans; safety monitoring; and more. Sifting through all sections takes time, and important details may be buried in fine print. Moreover, protocols use technical and regulatory language. It’s easy to lose the “big picture” in the details. Reviewers must strike a balance by skimming for overall design, then diving into critical parts like the objectives and eligibility. Skipping this careful reading risks missing flaws that could compromise safety or data quality. In fact, protocols are central to trial integrity, ensuring consistency across sites and adherence to ethical standards [1]. A focused, systematic reading strategy (aided by checklists and tools) is key to handling this complexity.
Check your trial design
When you begin reviewing a protocol, start with the broad overview and objectives. Look for a summary or schema (diagram) early in the document. The study objectives and rationale usually appear near the front. Read these carefully to understand the trial’s purpose. What medical question is being asked, and why it matters? Also note any sub-studies or additional aims listed. One guide recommends quickly checking if a protocol has many sub-studies and asking whether it’s feasible to do them all [3]. If the trial involves multiple community groups or sites, confirm that each population has been considered.
Next, examine the study design summary or schema [3]. This should outline the number of treatment arms, randomization, control groups, and key endpoints. The schema gives a “big picture” of the study. Verify that the design makes sense for the objectives. For example, if there is a placebo group or control arm, look for a clear rationale. Ensure the sample size seems adequate and that eligibility criteria are stated. A well-written protocol lists all inclusion and exclusion criteria and describes the study population clearly. Check that these criteria are scientifically justified and not overly restrictive. Unnecessary exclusions can make enrollment hard; ask if any groups are left out without good reason. Also see if the population description uses clear, non-stigmatizing language.
Other critical sections include procedures and schedule of visits. Protocols usually have a “Schedule of Events” detailing what happens at each study visit. Review this carefully: how many tests or questionnaires will participants undergo, and are they feasible? For instance, if hundreds of blood draws or scans are planned, that might be impractical. Check whether participant data (like lab results) will be returned promptly or “batched” later, which can affect safety monitoring. Note also the timeline. How long each participant stays in the study, and when results are expected. Understand when outcomes will be measured and when final data collection ends.
Don’t forget statistical analysis and endpoints. The protocol should describe primary and secondary endpoints (the main outcomes being measured). It should also outline the statistical plan (often in an appendix or Statistical Analysis Plan). A missing or vague statistical plan is a red flag. For example, the Clinical Trial Risk Tool specifically checks whether a Statistical Analysis Plan (SAP) is present. Trials lacking an SAP or using non-standard endpoints are flagged as higher risk. Make sure the number of participants is justified by a power calculation, and that the analyses will truly answer the study questions.
Finally, review safety, ethics, and consent information. Good protocols include a section on safety monitoring (adverse events reporting, data safety boards) and explain how risks to participants will be managed. The informed consent process should be outlined: the protocol often indicates how participants are informed of risks, benefits, compensation, and confidentiality. Check that consent will be in clear language and that participants’ rights (like withdrawing from the study) are respected. Ensuring this information is present and understandable is part of protocol review.
In summary, key sections to examine include:
Objectives and Rationale – What is the trial’s main goal? Are sub-aims reasonable.
Design and Schema – How many arms, randomization, blinding? Is there a clear control group?
Population and Eligibility – Who can join, and who is excluded? Are criteria inclusive and justified.
Procedures and Schedule – What happens at each visit? Are procedures feasible and results timely.
Endpoints and Analysis – What are the primary/secondary outcomes, and is a statistical analysis plan included.
Ethics and Safety – How will risks be explained and monitored? Is consent handled clearly.
Use the Table of Contents or Summary on page one to orient yourself. Some reviewers find it helpful to make a checklist of these sections or to jot margin notes while skimming. But above all, keep the big questions in mind: Does the protocol design match the objectives, and is the study likely to enroll participants safely and yield meaningful results?
Long protocols can be accelerated by technology. The Clinical Trial Risk Tool (CTRT) is an example of an AI-based assistant for protocol review. You upload the protocol text, and the tool automatically scans for hundreds of key features. It flags potential “red flags” that reviewers normally look for. For instance, it detects if the protocol lacks a detailed statistical analysis plan or uses unconventional endpoints. It also gathers data points like sample size, number of arms, disease area, and whether standard design elements (randomization, blinding) are present.
This AI tool helps users assess trials more rapidly and consistently than manual review. It literally saves time by doing the first-pass reading of technical details and highlighting where a human reviewer should look closer. For example, if the AI notes underpowering or design issues, you can focus your effort on questioning the sample size or endpoints. The tool even estimates the trial’s likely cost and can generate an itemized budget (based on the schedule of procedures). All of this makes the review more objective, since the same criteria are applied uniformly.
Reading a clinical trial protocol thoroughly is essential but need not be overwhelming. By following a structured approach – starting with objectives and design, then diving into eligibility, procedures, and safety – you cover the critical elements efficiently. Use checklists or headings as guides, and make notes of any uncertainties or gaps. Increasingly, AI-powered aids like the Clinical Trial Risk Tool can do the heavy lifting of scanning for technical items and potential flaws, letting you focus on interpretation and discussion. In combination, careful manual review and smart tools make the process faster, more objective, and ultimately more reliable.
Skim first, then focus. Get the big picture from the summary/schema before reading details.
Prioritize critical sections. Always check objectives, design, eligibility, endpoints, and safety.
Ask questions as you go. Is the trial answering a meaningful question? Are participants protected? Is the plan realistic?
Use technology. Tools like CTRT can highlight design issues and flag risks, reducing manual effort.
These strategies will help you digest even the longest protocols and ensure no important detail slips through the cracks.

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Upload your clinical trial protocol and create a cost benchmark with AI Protocol to cost benchmark The Clinical Trial Risk Tool uses AI and Natural Language Processing (NLP) to estimate the cost of a trial using the information contained in the clinical trial protocol.

You can download a white paper about clinical trial cost benchmarking here Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.