
We have developed a tool allowing researchers to analyse HIV and TB Clinical Trial Protocols and identify risk factors using Natural Language Processing. The tool allows a user to upload a clinical trial protocol in PDF format, and the tool will generate a risk assessment of the trial. You can find example protocols by searching on ClinicalTrials.gov.
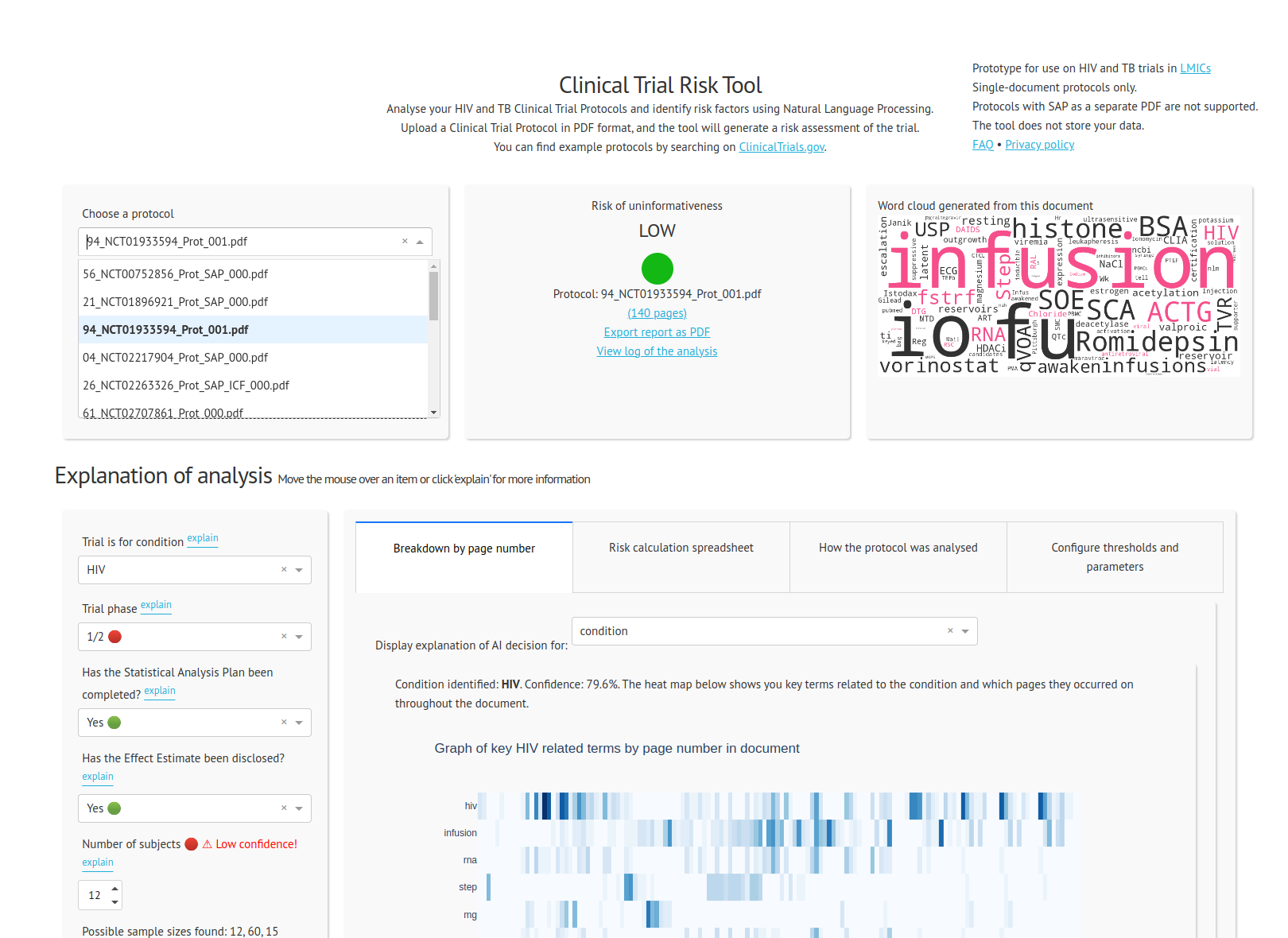
The tool allows a user to upload a trial protocol in PDF format. The tool processes the PDF into plain text and identifies features which indicate high or low risk of uninformativeness.
At present the tool supports the following features:
The features are then passed into a scoring formula which scores the protocol from 0 to 100, and then the protocol is flagged as HIGH, MEDIUM or LOW risk.
The Protocol Analysis Tool runs on Python and requires or uses the packages Plotly Dash, Scikit-Learn, SpaCy and NLTK. The tool runs as a web app in the user’s browser. It is developed as a Docker container and it has been deployed to the cloud as a Microsoft Azure Web App.
PDFs are converted to text using the library Tika, developed by Apache.
All third-party components are open source and there are no closed source dependencies.
A list of the accuracy scores of the various components is provided here.
Download this repository from the Github link as in the below screenshot, and unzip it on your computer
Alternatively if you are using Git in the command line,
Now you have the source code. You can edit it in your favourite IDE, or alternatively run it with Docker:
front_end. Run the command: docker-compose up
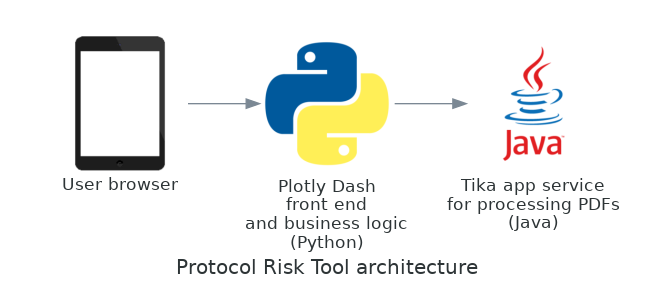
Each parameter is identified in the document by a stand-alone component. The majority of these components use machine learning but three (Phase, Number of Subjects and Countries) use a combined rule-based + machine learning ensemble approach. For example, identifying phase was easier to achieve using a list of key words and phrases, rather than a machine learning approach.
The default sample size tertiles were derived from a sample of 21 trials in LMICs, but have been rounded and manually adjusted based on statistics from ClinicalTrials.gov data.
The tertiles were first calculated using the training dataset, but in a number of phase and pathology combinations the data was too sparse and so tertile values had to be used from ClinicalTrials.gov. The ClinicalTrials.gov data dump was used from 28 Feb 2022.
Future development work on this project could include:
We have identified the potential for natural language processing to extract data from protocols at BMGF. Both machine learning and rule-based methods have a huge potential for this problem, and machine learning models wrapped inside a user-friendly GUI make the power of AI evident and accessible to stakeholders throughout the organisation.
With the protocol analysis tool, it is possible to explore protocols and systematically identify risk factors very quickly.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction As we move toward 2025, clinical trial regulations are undergoing significant transformation. This shift is being fueled by technological advancements, changing healthcare needs, and an increasing emphasis on transparency and patient safety. In this post, we will explore the key clinical trial regulations shaping the clinical trial landscape, the challenges professionals face, and the strategies they must adopt to navigate this ever-evolving environment.