
On 8 October, Thomas Wood of Fast Data Science presented the Clinical Trial Risk Tool, along with the Harmony project, at the AI and Deep Learning for Enterprise (AI|DL) meetup sponsored by Daemon. You can now watch the recording of the live stream on AI|DL’s YouTube channel below:
The Clinical Trial Risk Tool leverages natural language processing to identify risk factors in clinical trial protocols. The Clinical Trial Risk Tool is online at https://clinicaltrialrisk.org/tool.
Artificial Intelligence and Deep Learning for Enterprise is a meetup group in London dedicated to talks from people in the industry using developments in AI for exciting real world applications.
We initially developed the Clinical Trial Risk Tool to identify risk factors in HIV and TB protocols. Version 2 is coming soon, which will also make cost predictions (i.e. predict the cost of running a trial in dollars), and which will also cover further disease areas, such as Enteric and diarrheal diseases, Influenza, Motor neurone disease, Multiple sclerosis, Neglected tropical diseases, Oncology, COVID, Cystic fibrosis, Malaria, and Polio.
The project has been funded by the Bill and Melinda Gates Foundation and we have published a technical paper in the journal Gates Open Research:
The software is under MIT License, meaning that it is open source, and can be freely used for other purposes, both commercial and non-commercial, with no restrictions attached. The source code is on Github at https://github.com/fastdatascience/clinical_trial_risk.
[Fast Data Science]](https://fastdatascience.com/) is a leading data science consultancy firm providing bespoke machine learning solutions for businesses of all sizes across the globe, with a concentration on the pharmaceutical and healthcare industries.
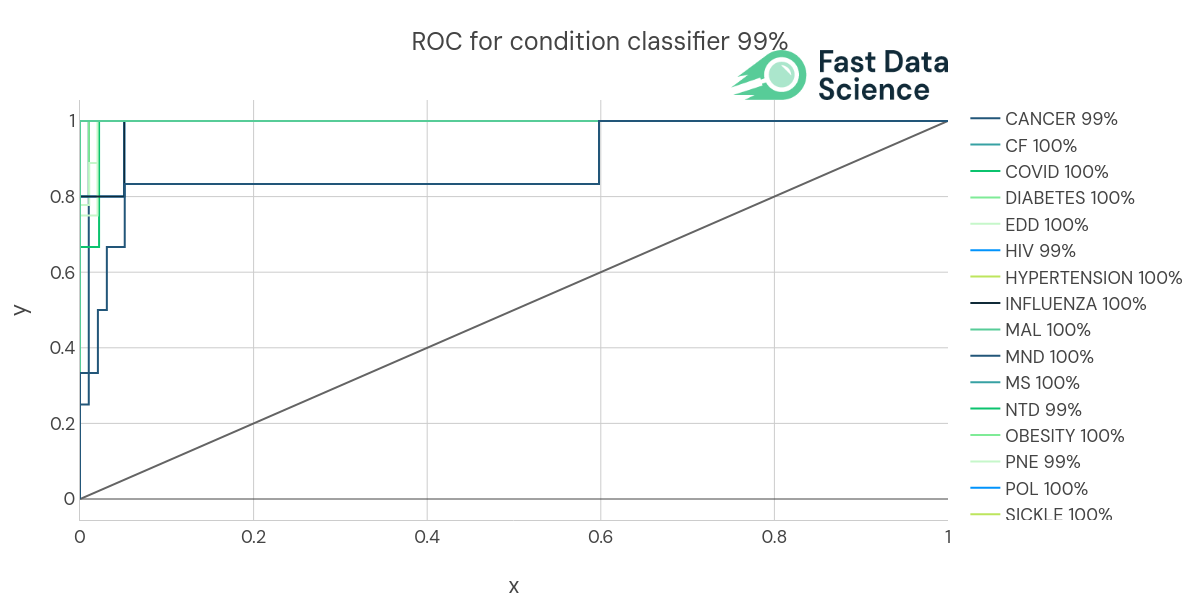
Introduction People have asked us often, how was the Clinical Trial Risk Tool trained? Does it just throw documents into ChatGPT? Or conversely, is it just an expert system, where we have painstakingly crafted keyword matching rules to look for important snippets of information in unstructured documents? Most of the tool is built using machine learning techniques. We either hand-annotated training data, or took training data from public sources. How We Trained the Models inside the Clinical Trial Risk Tool The different models inside the Clinical Trial Risk tool have been trained on real data, mostly taken from clinical trial repositories such as clinicaltrials.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.