Guest post by Youssef Soliman, medical student at Assiut University and biostatistician
In today’s complex research environment, managing the finances of a clinical trial is as crucial as managing the science. Dedicated clinical trial financial management software solutions have emerged to oversee the monetary aspects of studies, helping maintain tight budgetary control and regulatory compliance. These platforms typically encompass capabilities like budget creation, real-time expense tracking, automated payments to sites and vendors, and the generation of audit-ready financial reports. Effective oversight of trial finances is paramount for the integrity and success of any study; by providing enhanced visibility into expenditures, these tools facilitate informed decision-making and prevent costly overspending.
Historically, sponsors and CROs relied on manual spreadsheets and fragmented processes that were prone to error. The adoption of specialized software has since streamlined operations, improved accuracy, and reduced administrative burden on research teams. Financial management platforms ensure that trials stay on budget and compliant, which in turn attracts funding and maintains sponsor confidence. Below, we highlight five leading software solutions that are helping clinical trial sponsors, CROs, and research professionals bring order and insight to trial finances.
Medidata’s CTFM is an end-to-end financial management suite widely used by sponsors and CROs to plan and control trial budgets. Part of the Medidata clinical cloud platform, it provides comprehensive tools from initial study budget planning and site budget negotiations through to automating site payments [1]. In practice, CTFM enables teams to build a study budget with speed and consistency. Its Rave Grants Manager module offers benchmark-driven budgeting at granular levels (by therapeutic area, phase, region, etc.), allowing real-time scenario modeling and fair-market value comparisons. Once the budget is set, the software streamlines the critical process of site contracting. Instead of lengthy email exchanges and spreadsheets, automated workflows and dashboards guide sponsors through site budget negotiations, providing transparency and tracking so agreements are reached faster [1].
Upload your clinical trial protocol and create a budget with AI
Another standout feature of Medidata CTFM is its tight integration with operational data systems. For example, the platform’s Rave Site Payments module links to Medidata’s EDC (or other EDC systems) so that investigator payments are triggered automatically by verified study events in real time. This ensures sites are paid accurately and on schedule without manual invoice reconciliation, eliminating payment delays that can frustrate trial sites. A dedicated site portal further enhances transparency by letting investigators and site staff view their payment status, budget allocations, and even automate invoicing, leading to greater financial stability for sites. Throughout, sponsors retain full oversight: CTFM’s unified database combines clinical and financial data, powering real-time analytics and forecasting tools that support proactive decision-making.
IQVIA’s Clinical Trial Financial Suite is a unified, intelligent platform that streamlines every financial aspect of running a trial. It consolidates study budgeting, contracting, forecasting, and payments into a single AI-powered system [2], whereas many organizations previously juggled these tasks across separate tools. IQVIA leverages its massive industry data and technology expertise to make CTFS a one-stop solution: sponsors can quickly develop realistic investigator grant budgets using the GrantPlan module’s repository of 38 million negotiated data points (covering global fair-market cost benchmarks) [2]. Those budgets feed directly into CTFS’s contracting workflow, which uses AI to ingest clinical trial agreement terms and accelerate site contract generation and negotiation.
One of CTFS’s greatest endorsements is its widespread adoption. According to a recent industry survey, about 90% of top pharmaceutical companies use IQVIA’s financial management technology for their trials. This popularity stems from the platform’s proven ability to handle complexity at scale. For instance, CTFS’s Site Payments and Participant Payments modules ensure that thousands of investigators and patients can be paid on time, every time. The system integrates with study data sources to automatically trigger payments, and it provides real-time visibility into payment status for all stakeholders [2].
Finally, IQVIA’s solution offers rich analytics and compliance features (audit trails, dashboards, and alerts) so that sponsors can monitor financial health and regulatory compliance in a unified dashboard. Given its end-to-end scope and flexibility (available as SaaS software, an outsourcing service, or a hybrid), CTFS is often viewed as a gold standard for enterprise-level clinical trial financial management software that can adapt to organizations’ needs while delivering efficiency at scale.
For sponsors seeking a more specialized approach to trial budgeting and vendor management, Clinical Maestro by Strategikon Pharma has emerged as a powerful solution. This cloud-based platform focuses on the business operations of clinical trials – especially planning trial costs, managing CRO/service provider bids, and controlling contracts – with an emphasis on efficiency and data-driven decision making. Many sponsors struggle with budgeting and outsourcing using laborious Excel trackers and email threads, and this is precisely the pain point Clinical Maestro addresses. It provides an integrated suite of modules (Portfolio, Source, and Lead, among others) that collectively enable sponsors to handle everything from scenario planning and RFP creation to ongoing budget tracking and change order management within one system.
A hallmark of Clinical Maestro is its accuracy and speed in budget development. The platform leverages extensive benchmarking data and algorithms to produce highly accurate study budgets [3]. In practice, sponsors can input their trial assumptions and instantly see cost estimates that align closely with real-world prices, instilling confidence that budgets are neither overly optimistic nor inflated. Moreover, tasks that once took weeks can now be done in hours. Strategikon reports a 10× reduction in the time and effort needed to generate complete study budgets and RFPs by using Clinical Maestro’s automated workflows [3]. This efficiency not only speeds up trial initiation but also yields significant savings
Financial management in clinical trials isn’t just about budgeting. It’s also about executing payments efficiently. Greenphire is the leading specialist when it comes to automating clinical trial payments and improving site and patient satisfaction. Now part of Suvoda, Greenphire’s product suite is laser-focused on the payment workflows that traditional CTMS or finance systems often handle poorly. Its flagship offerings, eClinicalGPS and ClinCard, address two critical needs: getting investigative sites paid faster and reimbursing trial participants seamlessly.
Greenphire’s eClinicalGPS is an automated global site payment platform that replaces manual invoicing with a centralized portal, automates payment calculations, and integrates with study data systems to trigger payments based on completed visits or milestones. Meanwhile, ClinCard is an industry-leading solution for participant stipends – essentially a smart debit card system that allows patients to receive travel reimbursements or per-visit payments instantly, with full compliance and tracking [4]. By implementing these tools, sponsors and CROs drastically reduce the administrative burden of cutting checks or processing invoices across potentially hundreds of sites and patients.
The impact of Greenphire’s technology is evidenced by its widespread adoption and the feedback from research sites. As of 2024, Greenphire’s platforms have supported over 1,300 clinical studies, facilitating more than 7.6 million payments across nearly 80 countries. Sites consistently report that payment delays are a major pain point in trials, with one survey noting 82% of sites feel that slow sponsor payments negatively affect their operations. Greenphire also provides transparency dashboards so sites can see what payments are pending or processed, and sponsors can get portfolio-level financial reports and forecasting from the system.
By streamlining site payments and patient reimbursements, Greenphire fills a crucial niche in clinical trial financial management. It allows sponsors and CROs to execute the financial plan without friction, thereby improving site relationships, enhancing participant retention, and ultimately keeping the trial’s financial operations running smoothly in the background.
Finally, among the new wave of innovative solutions is our own Clinical Trial Risk Tool, which takes a unique AI-driven approach to financial management. Developed by Fast Data Science, this software was originally created to assess clinical trial risk, but it also includes a powerful cost modeling and budgeting module that sets it apart from traditional systems. The tool uses natural language processing (NLP) and machine learning to analyze the text of a clinical trial protocol and automatically estimate the trial’s costs [5].
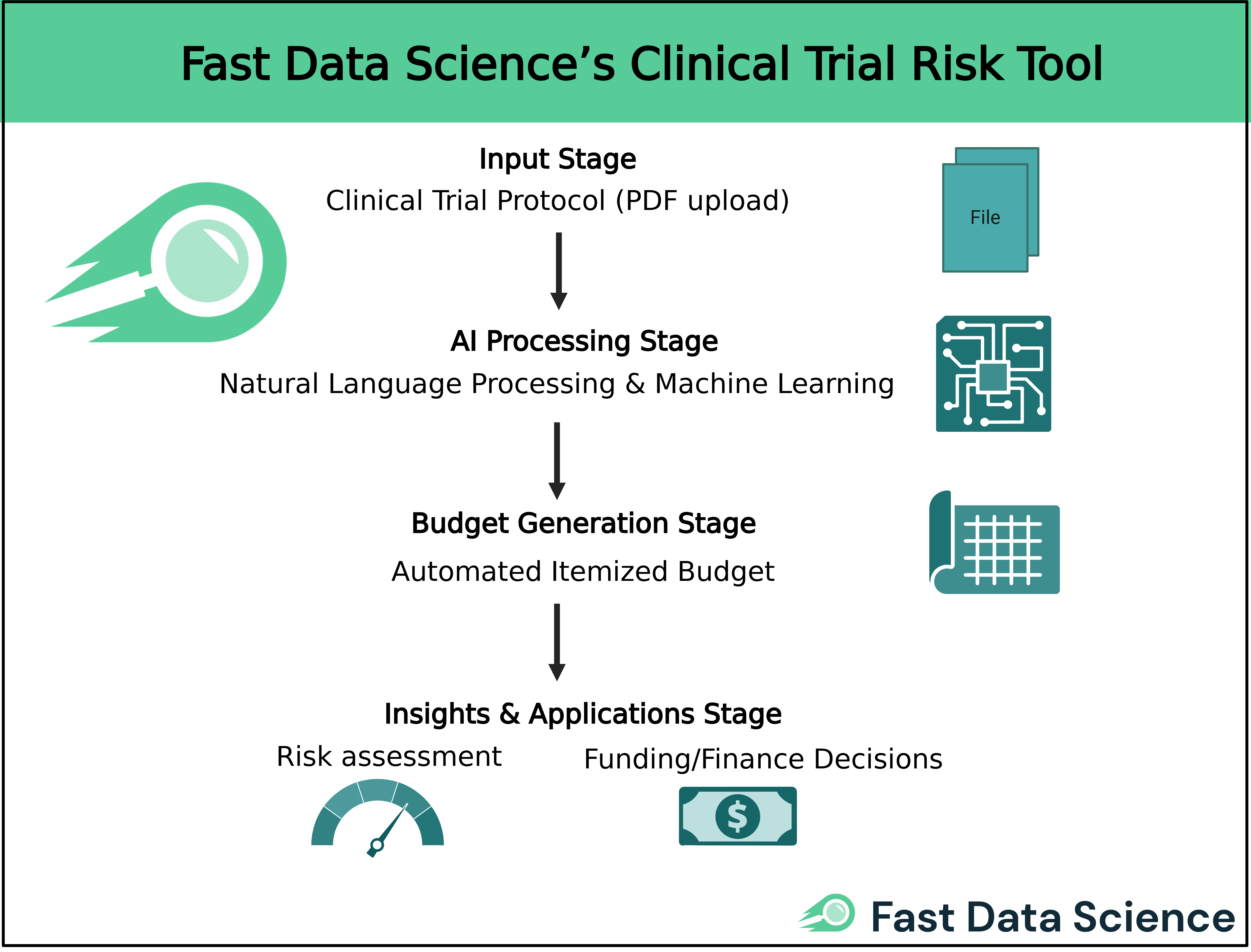
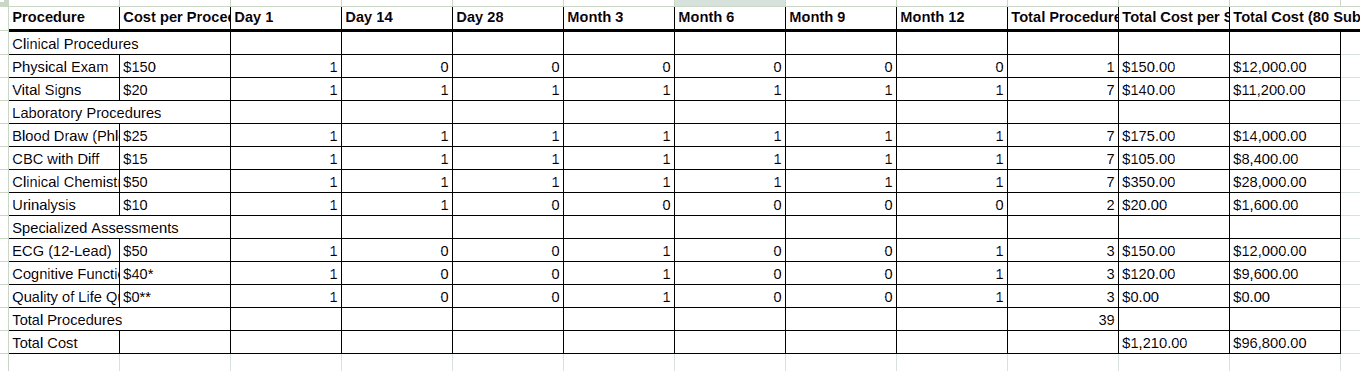
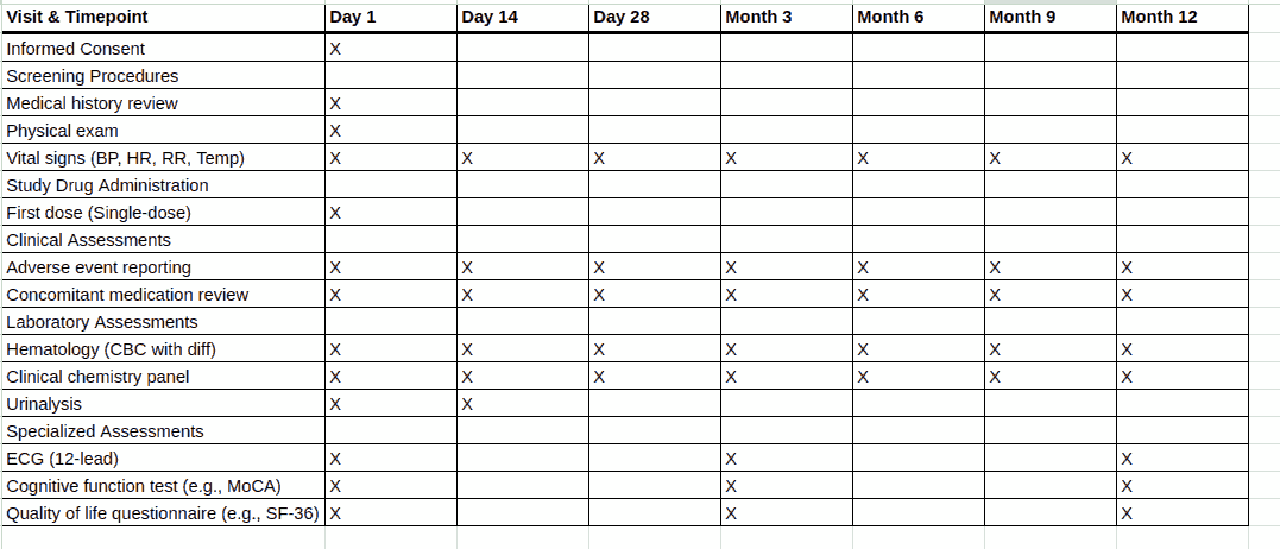
In other words, instead of manually building a budget from scratch, a sponsor can upload the protocol document (typically a PDF), and the Risk Tool’s algorithms will parse critical details like the Schedule of Events. The software then generates a multi-tabbed Excel budget that itemizes every procedure, visit, and operational cost for each study arm or site [5]. This bottom-up budgeting approach is enabled by AI models that have been trained on extensive historical trial data, allowing the system to recognize trial activities and assign appropriate cost benchmarks to them [5]. The convenience is tremendous. What might take a financial analyst days or weeks to manually budget (reading the protocol line-by-line to tally tests, treatments, monitoring, etc.) can be done in minutes by the AI, with a detailed itemized budget output ready for review.
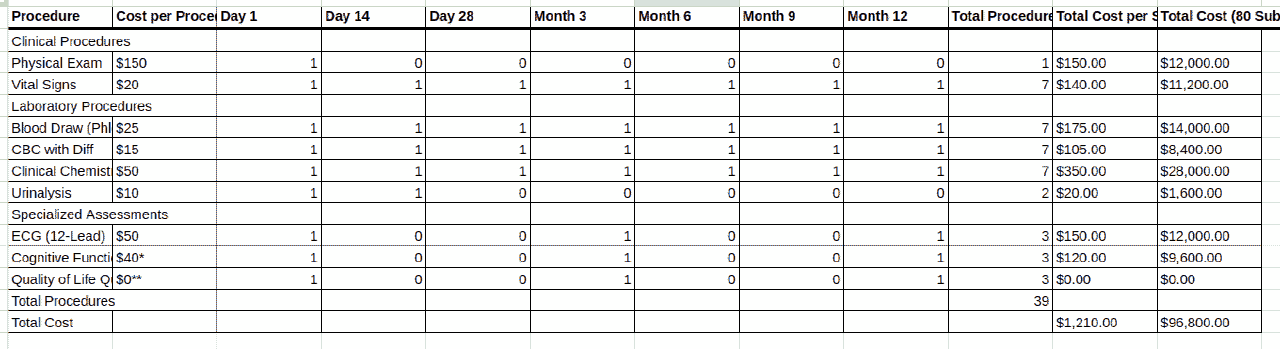
Moreover, the tool has garnered support from global health organizations. The Bill & Melinda Gates Foundation employs it to evaluate trial risks and costs in its funding decisions. The dual focus on risk and finance is a differentiator. By highlighting factors in the protocol that drive up risk or cost, the software helps study designers refine protocols for efficiency. For sponsors and CROs, Fast Data Science’s Clinical Trial Risk Tool represents a cutting-edge addition to the toolkit. It marries AI-driven insights with practical financial management, enabling teams to forecast trial costs with data-backed confidence and build detailed budgets without the usual drudgery. As trials grow more complex and data-rich, such intelligent automation ensures that budgeting and financial planning can keep pace, ultimately supporting better resource allocation and fewer budget surprises down the line.
Choosing the right clinical trial financial management software can make a profound difference in the success of a study. The five solutions profiled above each excel in different aspects. From comprehensive suites that cover end-to-end financial workflows, to specialized tools honing in on budget planning accuracy, payment automation, or AI-driven cost prediction. Clinical trial financial management software is not one-size-fits-all. A large global sponsor might prioritize an integrated platform like IQVIA CTFS or Medidata CTFM to handle thousands of payments and multi-currency budgets, whereas a lean biotech or an academic sponsor might value the precision of Clinical Maestro or the protocol-driven automation of the Clinical Trial Risk Tool.
What they all share, however, is the ability to replace inefficient manual processes with streamlined, transparent, and auditable financial management. By using modern financial management tools, clinical research professionals can mitigate avoidable budget risks, stay compliant with regulatory requirements, and ultimately re-focus their energy on what matters most. The investment in the right solution pays off in smoother trials today and better strategic planning for the trials of tomorrow.

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Upload your clinical trial protocol and create a cost benchmark with AI Protocol to cost benchmark The Clinical Trial Risk Tool uses AI and Natural Language Processing (NLP) to estimate the cost of a trial using the information contained in the clinical trial protocol.

You can download a white paper about clinical trial cost benchmarking here Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.