Clinical trials are the backbone of medical progress, but navigating their design and execution can be complex. Fast Data Science is dedicated to helping researchers by analysing clinical trial protocols through the power of Natural Language Processing (NLP).
We are presenting a selection of software which can be used for clinical trial protocol analysis or clinical trial cost prediction, forecasting, and risk assessment.
Fast Data Science’s Clinical Trial Risk Tool (https://clinicaltrialrisk.org/tool) allows researchers to upload a protocol as a PDF, and it uses an AI model to estimate the risk of the trial ending uninformatively which makes it a go-to clinical trial strategy software for many companies.
The Clinical Trial Risk Tool is free to use for pathologies in an international development context such as HIV and TB, Enteric and diarrheal diseases, Influenza, Neglected tropical diseases, COVID, Malaria, and Polio. For trials in Oncology, Motor neurone disease, Multiple sclerosis, Cystic fibrosis, and other pathologies, it will be available on a subscription basis.
Cost your clinical trial protocol
You can read more on our website about how the Clinical Trial Risk Tool works. The clinical trial protocol software tool was a winner of the Plotly Dash Apps Challenge and has been published and peer-reviewed in Gates Open Research and featured in the industry publication Clinical Leader.
This web-based clinical trial software platform facilitates the creation, management, and registration of high-quality protocols, particularly beneficial for researchers in low-resource settings (access through The Global Health Network: https://edctpknowledgehub.tghn.org/protocol-development/septre-protocol-tool/)
This SaaS platform targets the financial aspects of clinical trials, assisting biopharmaceutical companies, CROs, and service providers with budgeting, proposal development, and vendor management (https://strategikonpharma.com/clinical-maestro-2/)
SoftFormance provides tools for planning, managing, and tracking clinical study data, promoting efficiency and transparency throughout the research process (https://www.softformance.com/industries/software-for-clinical-trials/).
Gotrial CLINICA is a cloud-based data platform which integrates complex clinical development datasets such as clinical trial data, regulatory approvals, disease or hospital data - amongst others. Find CLINICA at https://www.gotrial.com/#clinica.
TrialPro is a free clinical trial software that allows you to import PDF protocols and analyses them on a cloud platform. You can create an account and try the tool for free at https://www.trialpro.ai/.
The Clinical Trial Participant Financial Burden Calculator is clinical trial financial management software designed to help potential clinical research participants/ patients better understand and estimate the financial impact and burden of participation. It doesn’t analyse protocols directly like our budgeting and financial modeling software, but you will need to enter the information about the trial manually. Find it at https://trialvalue-burdencalculator.com/.
OpentronsAI uses an interface powered by Generative AI and LLMs, so that scientists can describe experimental protocols in plain language, and OpentronsAI interprets and generates corresponding automated protocol scripts intelligently. Find it at https://opentrons.com/ai.
The Shiny CRT Calculator doesn’t analyse protocols directly but it serves as a tool to calculate power and sample sizes for Cluster Randomised Trials. Find it at https://clusterrcts.shinyapps.io/rshinyapp/.
A number of software tools exist for clinical trial data management.
The best clinical trial protocol analysis software for your research depends on your specific goals. Whether it’s risk assessment, or financial and capacity planning, a dedicated tool can significantly streamline your workflow.
Fast Data Science is committed to expanding our NLP capabilities to new disease areas. Stay tuned for future updates as we strive to empower researchers across a wider range of clinical trials!
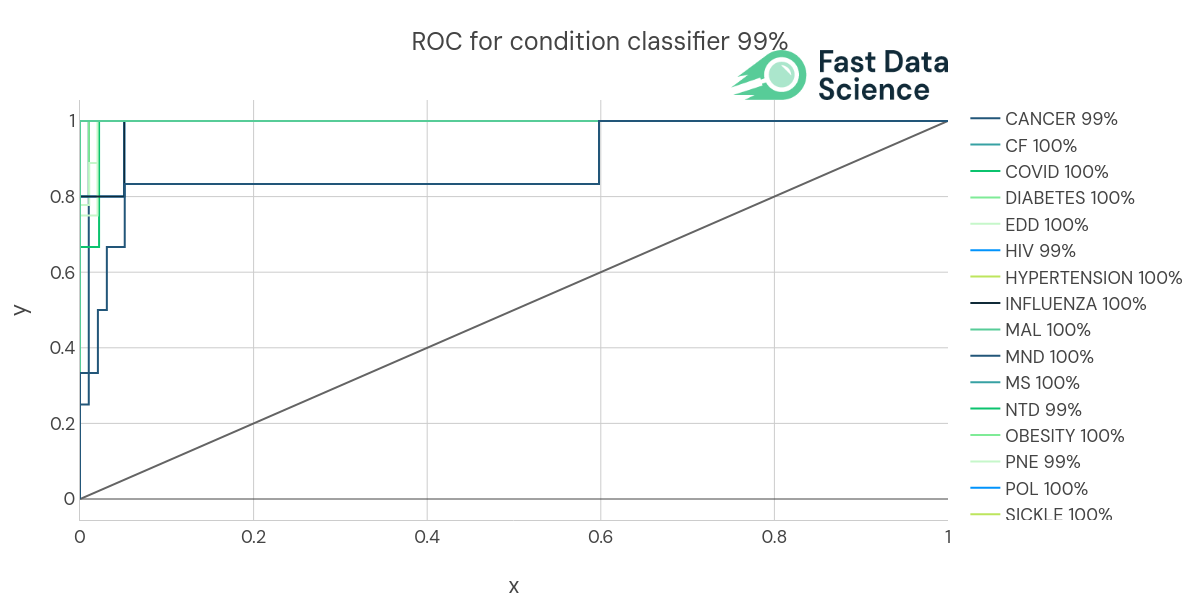
Introduction People have asked us often, how was the Clinical Trial Risk Tool trained? Does it just throw documents into ChatGPT? Or conversely, is it just an expert system, where we have painstakingly crafted keyword matching rules to look for important snippets of information in unstructured documents? Most of the tool is built using machine learning techniques. We either hand-annotated training data, or took training data from public sources. How We Trained the Models inside the Clinical Trial Risk Tool The different models inside the Clinical Trial Risk tool have been trained on real data, mostly taken from clinical trial repositories such as clinicaltrials.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.