Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan
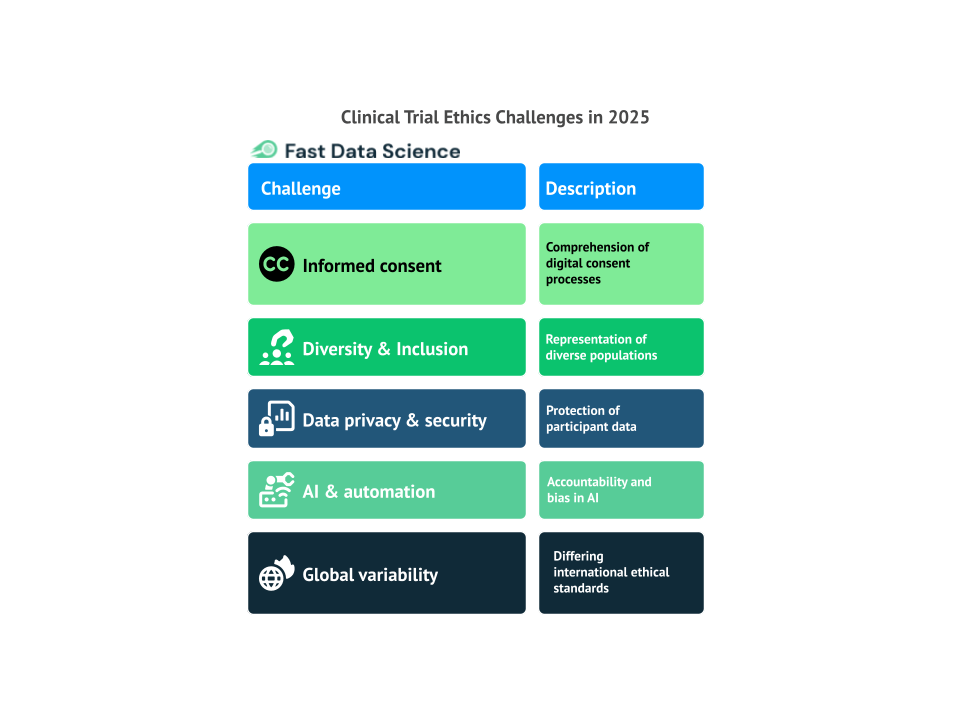
In 2025, clinical trials will continue to be a fundamental element in advancing medical science. Read more about: The Importance of Clinical Trials in Advancing Healthcare. However, as the landscape of medicine evolves, so do the ethical challenges that accompany these trials. The rapid progress of technology and the growing interconnectedness of the world present fresh ethical concerns that need to be tackled. This blog highlights the key ethical challenges that will confront clinical trials in 2025.
Informed consent has always been a crucial component of clinical trials. Traditionally, it involved paper forms or direct conversations with healthcare providers. However, as we move into 2025, the process of obtaining informed consent is evolving with the rise of digital health technologies such as wearables, AI-driven apps, and telemedicine.
While these advancements offer new methods for interacting with trial participants, they also introduce new challenges. One major concern is whether participants fully comprehend what they are agreeing to when digital tools mediate the consent process. For example, while apps or websites may provide all the necessary information, the likelihood of miscommunication increases when participants are left to make decisions without the personalized assistance of a healthcare professional.
Additionally, many digital health tools collect real-time data, which could be overwhelming or even intimidating for some participants. How can we ensure that participants fully understand how their personal data will be utilized, stored, and shared—particularly when much of this data involves sensitive health information? Maintaining the validity, clarity, and ethical integrity of informed consent will be a critical challenge in clinical trials moving forward into 2025.

The lack of diversity in clinical trials has been an ongoing ethical challenge. This gap in representation creates significant concerns, as it can lead to biased results that fail to capture how different groups respond to treatments. Research studies show that underrepresented populations remain largely excluded from clinical trials, which skews the data and limits the broader applicability of findings [1]. Without sufficient representation, the results of clinical trials may not reflect the experiences of the general population, putting certain groups at a disadvantage when it comes to healthcare. For this reason, the National Institutes of Health in The NIH approach to inclusive excellence has recommended setting specific inclusion goals for underrepresented populations, reflecting a move toward more systematic approaches to enhancing diversity in clinical research.
Looking ahead to 2025, ensuring diversity and inclusion in clinical trials will become even more crucial. As personalized medicine gains traction, it’s vital for clinical trials to accurately represent diverse populations. However, achieving this diversity is challenging, as various cultural, financial, logistical, and systemic obstacles continue to hinder participation from marginalized groups.
Addressing this challenge will require not only targeted recruitment efforts but also a fundamental change in how clinical trials are structured. It will be essential to ensure that clinical trials are inclusive, representative, and accessible to all individuals, regardless of their background or circumstances.
As clinical trials become more reliant on data, concerns about data privacy and security are at the forefront. By 2025, the collection, storage, and sharing of health data will be more extensive than ever, as electronic health records, wearable devices, and mobile apps generate vast amounts of real-time data. While these technological advances offer the potential to enhance patient care and streamline clinical trials, they also bring significant ethical challenges related to the protection of patient privacy.
One of the most pressing ethical issues in clinical trials today is ensuring the security of participants’ personal and medical data. As digital tools become integral to clinical trials, the risk of data breaches and unauthorized access increases. Patients may hesitate to provide sensitive information if they are unsure that their data will be kept safe or handled appropriately. Research has shown that many clinical trial participants have concerns about how their data is used, highlighting a trust gap between participants and researchers [2]. Meanwhile, trial sponsors and researchers are faced with the ethical challenge of balancing the need for transparency and data sharing with their responsibility to protect participants’ privacy.
In 2025, regulations governing data privacy will need to evolve to meet these new challenges. While frameworks like the European Union’s General Data Protection Regulation provide a foundational approach to data privacy, the growing complexity of clinical trial data demands even stricter safeguards.
Artificial intelligence (AI) and automation are increasingly playing a role in clinical trials, from data analysis and patient monitoring to clinical decision-making and trial recruitment. Read more about: Transforming Clinical Trials with Fast Clinical AI. While these technologies offer the potential to revolutionize the efficiency and accuracy of clinical trials, they also introduce several ethical concerns.
One of the most pressing issues is accountability. As AI systems take on more responsibilities within clinical trials, determining who is accountable when something goes wrong becomes more complex. For example, if an AI algorithm makes an erroneous recommendation that results in harm to a patient, who should be held responsible—the AI developers, the researchers, or the healthcare providers using the system? Ensuring clear accountability in AI-driven clinical trials will be one of the major challenges moving forward.
Another ethical dilemma is the potential for bias within AI algorithms. If the data used to train AI systems is flawed or unrepresentative, the algorithms may produce unfair or discriminatory outcomes. A survey of clinical trial professionals revealed that while many believe AI could significantly improve trial efficiency, there are still considerable concerns about the ethical implications of machine-generated decisions [3]. For example, AI systems might unintentionally prioritize certain demographic groups, thereby reinforcing existing healthcare disparities. The challenge here is ensuring that AI algorithms are transparent, equitable, and free from bias.
Finally, the increasing reliance on automation in clinical trials raises questions about the importance of human involvement in medical research. While AI can enhance efficiency, it cannot replace the critical role of human judgment, empathy, and oversight in clinical trials. Balancing the benefits of technology with the need for human oversight will be an ongoing ethical challenge in the clinical trials of 2025.
Check your trial design
Conducting clinical trials on a global scale is becoming increasingly common, with many trials involving participants from multiple countries. However, this global expansion presents significant ethical challenges, as different countries have different regulations and standards when it comes to clinical trial ethics. What is considered ethically acceptable in one country may not be in another, and this can create conflicts when conducting multinational trials. A comparative study found that not all countries follow the ethical standards set by the World Health Organization (WHO), raising concerns about participant safety and the integrity of data [4].
In such situations, researchers must grapple with the tough decision of whether to proceed with a trial in a country where ethical standards are lower or to seek participants from countries with stricter ethical protections. The ethical dilemma here is how to ensure that participants are treated fairly and equitably, no matter where the trial takes place.
Furthermore, cultural differences can influence how clinical trials are viewed and how participants are approached. Some cultures may prioritize collective decision-making, while others place greater value on individual autonomy. Managing these cultural differences while maintaining a consistent ethical approach across international trials will be one of the biggest challenges for global clinical research in 2025.
As we approach 2025, the ethical landscape of clinical trials is becoming more intricate. Issues such as informed consent in a digital age, diversity and inclusion, data privacy, the growth of AI, and differences in global standards will require clinical trial ethics to adapt to both technological progress and societal shifts. By tackling these challenges directly, we can ensure that clinical trials in 2025 and beyond are carried out in a manner that upholds patient rights, ensures fairness, and strengthens trust in the clinical research process.
Budiyanti, R.T., et al., Integration of Electronic Medical Record and SATUSEHAT’s Platform: Patient’s Legal Protection Perspective. I-HeLis, 2023. 1(2): p. 1-8.
Gupta, R., et al., Consumer Views on Privacy Protections and Sharing of Personal Digital Health Information. JAMA Network Open, 2023. 6(3): p. e231305.
Tanaka, Y., Occupational Medicine, Go Above and Beyond. Journal of UOEH, 2024. 46(1): p. 79-86.
Doan, X., et al., Comparing Attitudes Toward Different Consent Mediums: Semi structured Qualitative Study. JMIR Human Factors, 2024. 11: p. e53113.

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Upload your clinical trial protocol and create a cost benchmark with AI Protocol to cost benchmark The Clinical Trial Risk Tool uses AI and Natural Language Processing (NLP) to estimate the cost of a trial using the information contained in the clinical trial protocol.

You can download a white paper about clinical trial cost benchmarking here Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.