
Guest post by Youssef Soliman, medical student at Assiut University and biostatistician
Designing a high-quality clinical trial protocol is critical for the success of any study. A protocol is the blueprint that outlines every aspect of a trial. In an ideal world, a flawless protocol would require no revisions and include only essential elements. In reality, however, the average protocol undergoes 2–3 amendments and often contains excessive data collection and overly complex entry criteria. Such protocol design for clinical trial complexity can lead to delays, increased costs, and recruitment challenges. To avoid these pitfalls, researchers and sponsors must invest time and care in developing clinical trial protocol documents that are thorough, clear, and compliant with guidelines.
Above: the Clinical Trial Risk Tool lets you upload a protocol and will automatically check the protocol design against its checklist, as well as generating a site budget for you.
Regulatory and ethical standards underscore the importance of a well-crafted protocol. Global Good Clinical Practice (GCP) guidelines (ICH E6) explicitly list the topics a protocol should cover [1]. Thus, when developing a clinical trial protocol, one must ensure all these elements are addressed. In summary, designing a protocol should be a systematic and evidence-based process, balancing scientific rigor with practical feasibility.
Check your trial design
Below we present a structured checklist and discussion of each key component in designing a protocol. This clinical trial protocol checklist retains all major sections, providing guidance on what information to include.
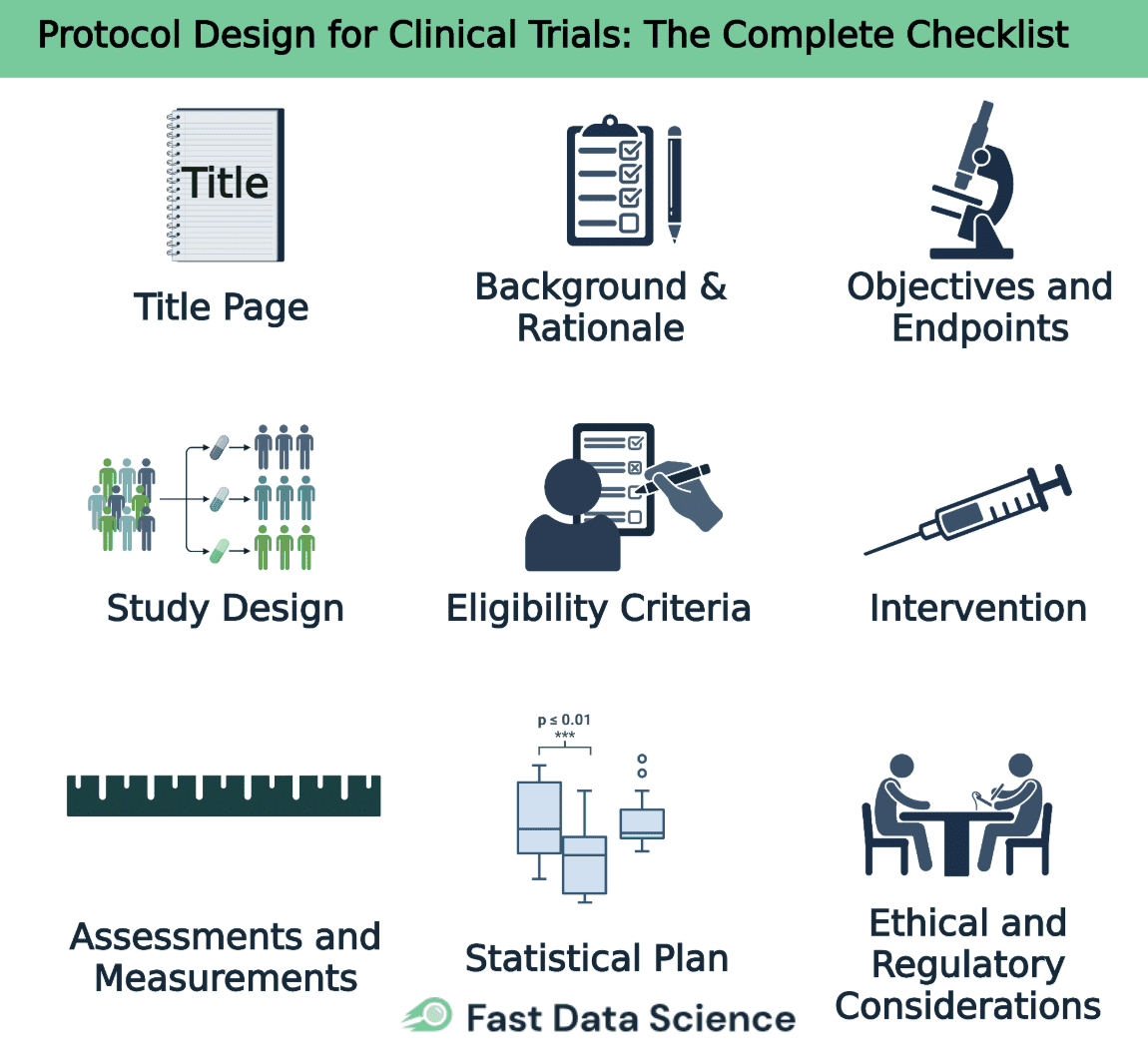
The title page should contain the protocol title, a unique protocol identification number or code, and the date (with a version number if applicable). It also lists the sponsor’s name and address, as well as key contact information for the sponsor’s medical expert and the investigator(s) responsible for the trial. Many protocols additionally name the protocol authors, contributors, and the site(s) or institutions involved. Crucially, each protocol version must be clearly dated and any amendments should be numbered for traceability.
This section provides the scientific background and rationale for the trial. It should summarize relevant preclinical findings and any prior clinical data that support the research, including known and potential risks and benefits of the investigational product or intervention. Clearly explain the medical problem being addressed and the gap in knowledge that the study aims to fill. All claims and motivations should be backed by references to the scientific literature and existing data.
State the trial’s objectives and hypotheses clearly. Define the primary objective – the main question the study seeks to answer – and any secondary objectives addressing additional questions. For each objective, specify the corresponding endpoint (outcome measure) that will be used to determine if the objective is met. Well-defined objectives and endpoints keep the protocol focused and guide all other aspects of the study design.
Describe the type of trial and overall study design. State the design framework (for example, a randomized controlled trial with parallel groups, double-blind, placebo-controlled) and the allocation ratio of participants to each arm. It’s often helpful to include a schematic diagram of the trial flow, showing stages such as recruitment, intervention, and follow-up. Explain any measures to minimize bias, such as the randomization method and blinding procedures, and note if there are any planned interim analyses, stopping rules, or adaptive design features. The protocol design must ensure the study’s scientific integrity and the credibility of the data produced.
Specify clear inclusion and exclusion criteria that define who is eligible (and who is not) to participate in the trial. These criteria typically cover demographics (e.g. age, sex), medical conditions (diagnosis, disease stage, health status), and other factors like prior treatments or concomitant illnesses. Well-chosen eligibility criteria ensure the study population is appropriate for the research question and help produce reliable, generalizable results [2]. Avoid making the criteria so restrictive that recruitment becomes difficult, unless those restrictions are scientifically justified. This section should also outline any criteria or procedures for withdrawing participants from the trial (for example, if their condition worsens or if they choose to withdraw).
Detail the interventions for each study arm (for example, the investigational drug, an active comparator, and/or a placebo). For each intervention, provide the dosing information including the dosage amount, frequency of administration, route of administration (e.g. oral, intravenous), and the duration of treatment. Explain how and when the treatments will be administered at each study visit, and describe any procedures for randomizing or allocating participants to their respective groups. Also specify any concomitant medications or supportive therapies that are permitted or prohibited during the trial. Providing clear, thorough instructions in this section ensures consistency in how the protocol is implemented across all study sites.
Provide a detailed plan for all efficacy and safety assessments that will be conducted. Define each outcome measure (endpoint) and how it will be measured, distinguishing between primary endpoints (for the primary objective) and secondary endpoints. For each assessment, specify when it will take place (e.g. at baseline, weekly, at the end of treatment) and the methods or instruments that will be used. The protocol should also describe safety monitoring in detail. For instance, what vital signs or laboratory tests will be done and how adverse events will be collected and reported. It is often useful to include a schedule of events table that maps out all visits and assessments over the course of the study.
Explain the statistical methods that will be used to analyze the data and evaluate the study objectives. Specify the planned statistical tests or models for the primary endpoint and for key secondary endpoints, and include details of any interim analysis plans if applicable. State the targeted number of participants (sample size) for the trial and provide the justification for this number, typically by summarizing a power calculation that shows the trial is adequately powered to detect a meaningful effect. Indicate the significance level (alpha) for hypothesis testing and describe any stopping rules or boundaries for early termination of the trial (such as for efficacy or futility). Additionally, outline how protocol deviations or missing data will be handled in the analysis. Involvement of a biostatistician in drafting this section is highly recommended to ensure a robust protocol design.
Describe how the trial will uphold ethical standards and comply with regulatory requirements. Include a statement that the study will be conducted in accordance with the protocol, ICH-GCP guidelines, and applicable regulatory requirements. Document that approval will be obtained from an Institutional Review Board (IRB) or Ethics Committee prior to initiating the trial, and that all participants (or their legal representatives) will provide informed consent. Discuss any special ethical considerations relevant to the study (for example, involving a vulnerable population or use of a placebo control) and how such issues will be addressed.
Outline how participant safety will be monitored during the trial. For instance, specify whether an independent Data Safety Monitoring Board (DSMB) will oversee interim data, and describe how serious adverse events will be reported. The protocol should also state that investigators will permit trial-related monitoring, audits, and inspections by the sponsor and regulatory authorities, providing direct access to source data/documents. Describe measures to protect participant privacy and data confidentiality (in compliance with regulations such as HIPAA, if applicable). Additionally, note whether the trial will be registered in a public trials registry (e.g. ClinicalTrials.gov) and outline plans for disseminating the study results. Ensuring protocol design for clinical trial compliance with all ethical and regulatory standards is crucial to protect participants and preserve data integrity.
By adhering to the above clinical trial protocol checklist of essential elements, investigators can develop a comprehensive and well-organized protocol. Indeed, international guidance such as the SPIRIT 2013 checklist is specifically aimed at ensuring that trial protocols address all key issues [3]. High-quality protocols that follow these principles not only meet regulatory and ethical expectations but also facilitate smooth trial conduct, minimizing confusion, deviations, and unnecessary amendments.
Figure 1. Checklist for protocol design of clinical trials.
Even with a strong checklist, there are recurrent pitfalls in protocol development that can undermine a study. Below are some common mistakes made when designing a protocol and strategies to avoid them:
Unfocused or Overambitious Objectives: Packing too many objectives or endpoints into one trial can overcomplicate the design and data analysis. Avoid this pitfall by prioritizing a clear primary objective (and a few secondary ones at most), thereby keeping the study focused and feasible [4]. Ensure each objective is tied to a specific endpoint and scientific rationale.
Overly Restrictive Eligibility: Excessively narrow inclusion/exclusion criteria (e.g. too many exclusion factors) may make it “almost impossible to enroll in a timely fashion” [4]. While criteria should protect patient safety and define the target population, avoid needless restrictions. Design criteria with real-world populations in mind, and conduct feasibility assessments to confirm that enough participants will qualify.
Inconsistent or Copy-Pasted Details: Reusing text from previous protocols or duplicating information in multiple sections can introduce contradictions or irrelevant requirements. For example, boilerplate entry criteria or assessments that don’t make sense for the current study will increase confusion. Avoid this by tailoring every section to the present trial and cross-checking for consistency. Have a fresh set of eyes (or an interdisciplinary team review) to catch any discrepancies.
Lack of Stakeholder Input: A protocol merges scientific, medical, regulatory, and operational perspectives. If all relevant experts (investigators, statisticians, clinicians, operations staff, etc.) are not involved in its development, important perspectives may be missed. Indeed, it is noted that too often protocol writers “go it alone” and overlook input from key departments [4]. Avoid this by seeking interdisciplinary input early. Engage team members to review and agree on the objectives, endpoints, and procedures before finalizing the protocol.
Ignoring Feasibility and Complexity: A design that looks good on paper may falter in practice if it’s too burdensome or unrealistic. Common issues include overly frequent assessments, complex logistics, or endpoints that require impractical measurements. Avoid this by conducting a thorough feasibility. Simplify the protocol where possible without compromising scientific integrity. A leaner, pragmatic design is more likely to be executed well and completed on time.
In conclusion, developing clinical trial protocol documentation is a balancing act between scientific ambition and practical execution. By adhering to a structured checklist of essential elements and staying vigilant about common pitfalls, clinical researchers and sponsors can create protocols that are both comprehensive and efficient. A well-designed protocol not only satisfies regulatory and ethical standards [5] but also sets the stage for a successful trial. It answers the research question, protects participants, and generates reliable results without unnecessary delays or amendments. With careful protocol design for clinical trial planning and attention to detail, the protocol becomes a powerful tool that guides the study smoothly from start to finish.
European Medicines Agency (ema.europa.eu), ICH E6 Guideline for Good Clinical Practice (Section 6: Protocol and Amendments)
Lindus Health (lindushealth.com), The Ultimate Guide to Designing Clinical Trial Protocols
SPIRIT 2013 Statement: Guidance for Clinical Trial Protocols
Allucent (allucent.com), Top Five Mistakes in Clinical Protocol Design
U.S. Food and Drug Administration (fda.gov), IND Applications for Clinical Investigations: Clinical Protocols

Estimating the total cost of a clinical trial before it runs is challenging. Public data on past trial costs can be hard to come by, as many companies guard this information carefully. Trials in high income countries and low and middle income countries have very different costs. Clinical trial costs are not normally distributed.[1] I took a dataset of just over 10,000 US-funded trials. You can see that the range is huge, from small device or behavioural trials costing as little as $50,000, while large multi-centre international trials can cost hundreds of millions.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of clinical studies relies heavily on proper financial planning and budgeting. These processes directly impact key factors such as project timelines, resource allocation, and compliance with regulatory requirements. The accurate forecasting of costs for clinical trials, however, is a highly complex and resource-intensive process. A study by the Tufts Center for the Study of Drug Development found that the average cost of developing a new drug is approximately $2.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction Recent years have seen a substantial rise in oncology clinical trials, with annual growth exceeding 260 studies on average [1]. Despite this increase, these studies continue to be some of the most demanding and resource-intensive in clinical research. The combination of intensive monitoring, detailed assessment schedules, and highly specific eligibility criteria creates substantial operational challenges.