
Guest post by Youssef Soliman, medical student at Assiut University and biostatistician Designing a high-quality clinical trial protocol is critical for the success of any study. A protocol is the blueprint that outlines every aspect of a trial. In an ideal world, a flawless protocol would require no revisions and include only essential elements. In reality, however, the average protocol undergoes 2–3 amendments and often contains excessive data collection and overly complex entry criteria.
Clinical trials have long been the foundation of medical breakthroughs, but traditional methods often stumble over slow timelines, high costs, and difficulties in finding the right participants. Artificial intelligence (AI) — a technology ready to transform this landscape by making trials faster, more affordable, and smarter. The accelerating adoption of AI in clinical trials signals a major shift in healthcare research. It is already making significant strides in transforming clinical trials.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan In clinical trials, a staggering 80% encounter delays during the startup phase and 37% struggle to meet enrollment targets. Read more Key clinical trial statistics. These figures highlight a critical, yet often underemphasized, aspect of clinical trials—the feasibility process. The feasibility process is essential for assessing the practicality of a clinical trial’s design, ensuring the study is prepared to tackle the challenges that may arise.

The key advantage of adaptive clinical trial design is the convenience and flexibility of prospective planning opportunities to modify the study design, test hypotheses based on interim results and control or eliminate bias, which is not possible in conventional clinical trial design. For example, based on interim data, a model-based approach could be employed to modify or optimize Phase 2 clinical trial doses based on patient’s dose-response. This approach could eliminate suboptimal dose risk and improve the drug efficacy rate without any safety signals.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Clinical trials are fundamental for generating the data needed to develop new treatments, ultimately improving patient care and advancing healthcare systems worldwide (you can read more in our recent post about the Importance of Clinical Trials). However, despite their essential role, these trials are often financially burdensome, which can hinder research efforts and limit patient access to innovative therapies.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Clinical trials are the backbone of clinical drug development. They are essential for advancing medicine, testing new therapies, and ensuring patient safety before drugs reach the market. Yet, despite enormous investment, approximately 90% of all clinical trials fail to achieve regulatory approval, according to recent industry studies.[1] Failed trials result in significant financial losses — the average cost of a failed Phase III trial alone can exceed $100 million.

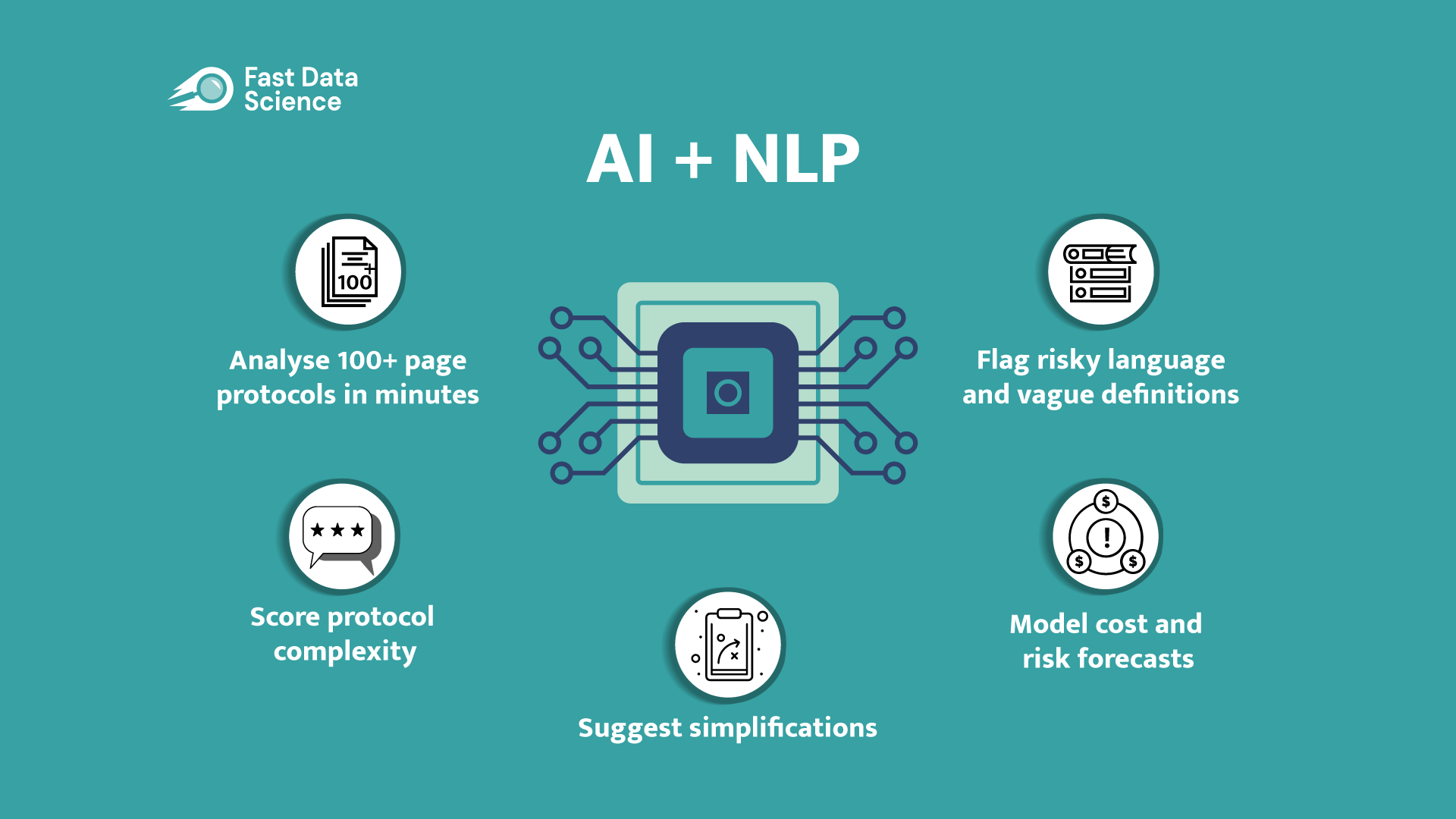
This post originally appeared on Fast Data Science’s blog on LinkedIn. Clinical trials are essential for medical advancement but are not without risk. Delays, budget overruns, and compliance issues can derail the most carefully planned studies. Proactive risk management is the key to ensuring patient safety, maintaining regulatory compliance, and achieving successful trial outcomes. In this article we’ll explore the key risks in clinical trials, how AI-powered tools like the Clinical Trial Risk Tool can help mitigate these risks, and practical strategies for ongoing risk monitoring.

This post originally appeared on Fast Data Science’s blog on LinkedIn. Discover how the Clinical Trial Risk Tool helps optimise clinical trial workflows with accurate risk and cost analysis. Save time and reduce costs. Why Workflow Efficiency Matters in Clinical Trials Running a clinical trial is a complex and expensive process. Delays, unexpected costs, and inefficiencies can waste time and money, affecting trial outcomes and patient care. As trials become more complicated, workflow management is more important than ever.

This post originally appeared on Fast Data Science’s blog on LinkedIn. In today’s ever-evolving healthcare landscape, technology is crucial to improving patient care, streamlining processes, and enhancing outcomes. Natural Language Processing (NLP) is one such technology that is revolutionising the way healthcare organisations operate. For instance, NLP has been used to analyse patient feedback and identify trends in satisfaction levels, leading to targeted improvements in service quality. In this article, we will explore the role of NLP in healthcare, its benefits, and potential applications.

This post originally appeared on Fast Data Science’s blog on LinkedIn. Clinical trials are vital for advancing medical innovation, yet they often face significant hurdles, including ensuring patient safety, adhering to regulatory requirements, controlling costs, and maintaining efficiency. Traditional risk assessment methods frequently need to be revised to address these complexities. Artificial Intelligence (AI) is transforming clinical trial management, offering data-driven solutions to predict and mitigate risks. AI-powered tools like the Clinical Trial Risk Tool have revolutionised trial planning and execution.