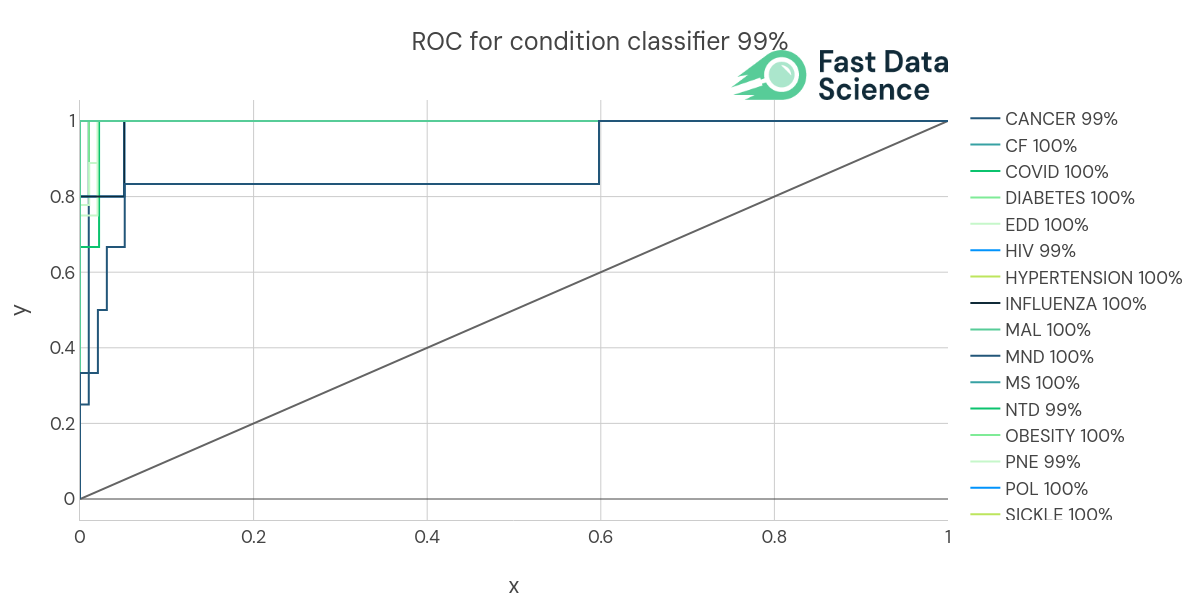
Introduction People have asked us often, how was the Clinical Trial Risk Tool trained? Does it just throw documents into ChatGPT? Or conversely, is it just an expert system, where we have painstakingly crafted keyword matching rules to look for important snippets of information in unstructured documents? Most of the tool is built using machine learning techniques. We either hand-annotated training data, or took training data from public sources. How We Trained the Models inside the Clinical Trial Risk Tool The different models inside the Clinical Trial Risk tool have been trained on real data, mostly taken from clinical trial repositories such as clinicaltrials.

Over the years, the overall cost of the drug development process has been exponentially increasing, prompting the adoption and use of adaptive clinical trial design software. Though there are practical difficulties and barriers in implementing clinical trial solutions, these problems are adequately addressed to overcome these issues as they arise. With advancements in software technologies, further improvements are being made to the software’s adaptive clinical trial design. Despite these progresses, just only a handful of well-established software with various types of clinical trial adaptations is currently available.

A clinical trial protocol is a document which serves as the step-by-step playbook for running the trial. The clinical trial protocol guides the study researchers to run the clinical trial effectively within a stipulated period. The prime focus of the clinical trial protocol is to ensure patients’ safety and data security. [1, 2] As the clinical trial protocol is an essential document for the seamless execution of the clinical trial, reviewing (peer-reviewing) the protocol is essential to ensure the scientific validity/viability/quality of the protocol.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction As we move toward 2025, clinical trial regulations are undergoing significant transformation. This shift is being fueled by technological advancements, changing healthcare needs, and an increasing emphasis on transparency and patient safety. In this post, we will explore the key clinical trial regulations shaping the clinical trial landscape, the challenges professionals face, and the strategies they must adopt to navigate this ever-evolving environment.
Thomas Wood has recently joined the Clinical Trial Files podcast with Karin Avila and Taymeyah Al-Toubah, discussing the inception of the Clinical Trial Risk Tool, what impact AI can make in clinical trials, and what Alan Turing would make of it all. This is an episode dedicated to Alan Turing’s 113th birthday on 23 June 2025. You can find the episode on Spotify Apple Podcasts Amazon Music Podcast Index Fountain Podcast Addict Podverse.

Guest post by Youssef Soliman, medical student at Assiut University and biostatistician Before launching a clinical study, even the most promising idea must be vetted for feasibility. In other words, can this trial be executed successfully in the real world? Feasibility assessments examine practical factors like available patients, site capabilities, timelines, and budget. This step is crucial. A majority of trials encounter delays or enrollment shortfalls (by some estimates, 70–80% of trials) [1], driving up costs and risking failure.

Risk assessment is a critical process in clinical trials that involves thorough and systematic evaluation of potential risks to the study volunteers, biases affecting the results and data integrity/protection. The process requires the use of risk assessment forms to identify, evaluate, and mitigate risks and ensure patient safety and data integrity.[1] The scope of this article is to discuss whether the utilization of clinical trial assessment templates is worth the time of the study sponsors/investigators.
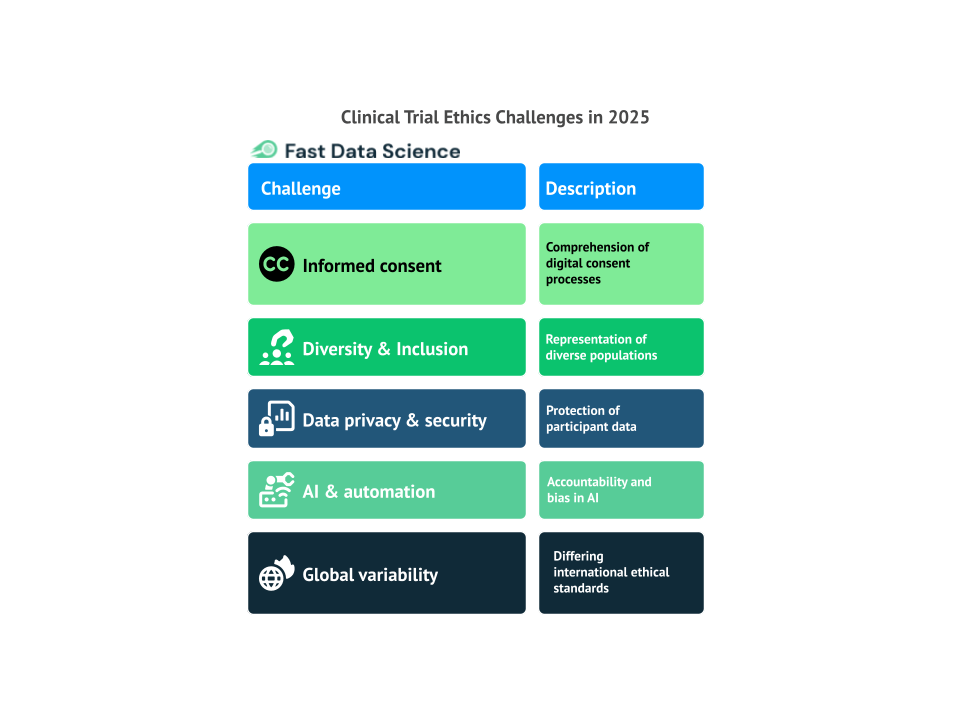
Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan In 2025, clinical trials will continue to be a fundamental element in advancing medical science. Read more about: The Importance of Clinical Trials in Advancing Healthcare. However, as the landscape of medicine evolves, so do the ethical challenges that accompany these trials. The rapid progress of technology and the growing interconnectedness of the world present fresh ethical concerns that need to be tackled.

Guest post by Youssef Soliman, medical student at Assiut University and biostatistician Introduction Conducting a clinical trial risk assessment is now a regulatory expectation and a cornerstone of quality management in clinical research. A risk assessment is a systematic process for identifying and evaluating events that could affect the achievement of a trial’s objectives [1]. In practice, this means examining the protocol, procedures, and trial environment to spot hazards to patient safety, data integrity or compliance.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Clinical trials are essential for advancing medical science, yet they are inherently complex and involve a wide range of risks. As a result, effective risk management in clinical trials is crucial to ensuring their successful completion. Among the various approaches to managing these risks, clinical trials Key Risk Indicators (KRIs) have become essential tools. KRIs are precise, measurable metrics that serve as early alerts for potential risk exposures in clinical studies.