How can you use the Clinical Trial Risk Tool to create a per-subject budget from a protocol or synopsis and a site Charge Master? The video below walks you through how the Clinical Trial Risk Tool by Fast Data Science can accelerate your budgeting. The Clinical Trial Risk Tool streamlines the creation of a per-subject budget by automating the typically manual process of extracting data from the Study Protocol and cross-referencing it with Charge Master/Fee Schedules.
Guest post by Youssef Soliman, medical student at Assiut University and biostatistician In today’s complex research environment, managing the finances of a clinical trial is as crucial as managing the science. Dedicated clinical trial financial management software solutions have emerged to oversee the monetary aspects of studies, helping maintain tight budgetary control and regulatory compliance. These platforms typically encompass capabilities like budget creation, real-time expense tracking, automated payments to sites and vendors, and the generation of audit-ready financial reports.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan* Introduction Pilot studies are a cornerstone of modern clinical research. These preliminary trials allow researchers to assess the feasibility of their hypotheses, refine methodologies, and identify potential obstacles before embarking on larger, more expensive trials with significant ethical implications. A well-structured pilot study not only saves time and resources but also reduces the chances of costly failures during later phases of the clinical trial process.

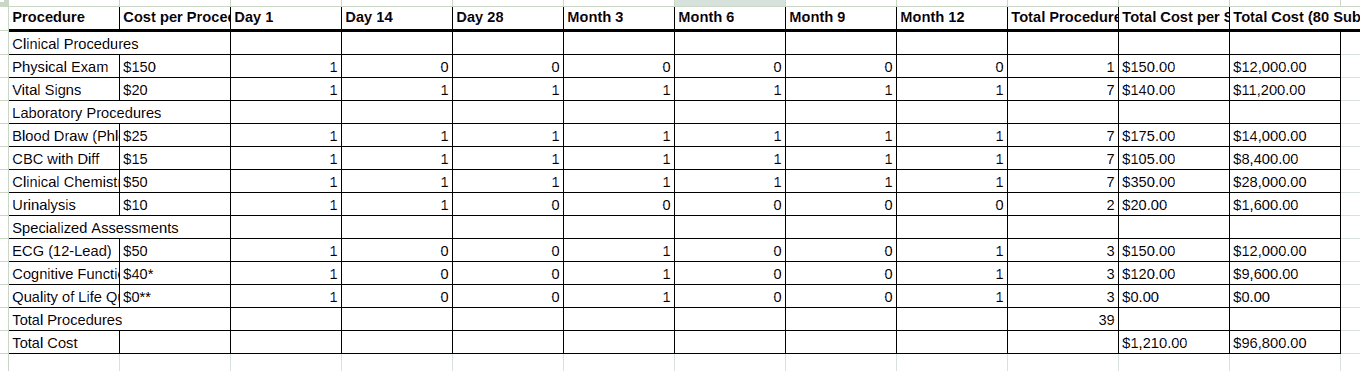
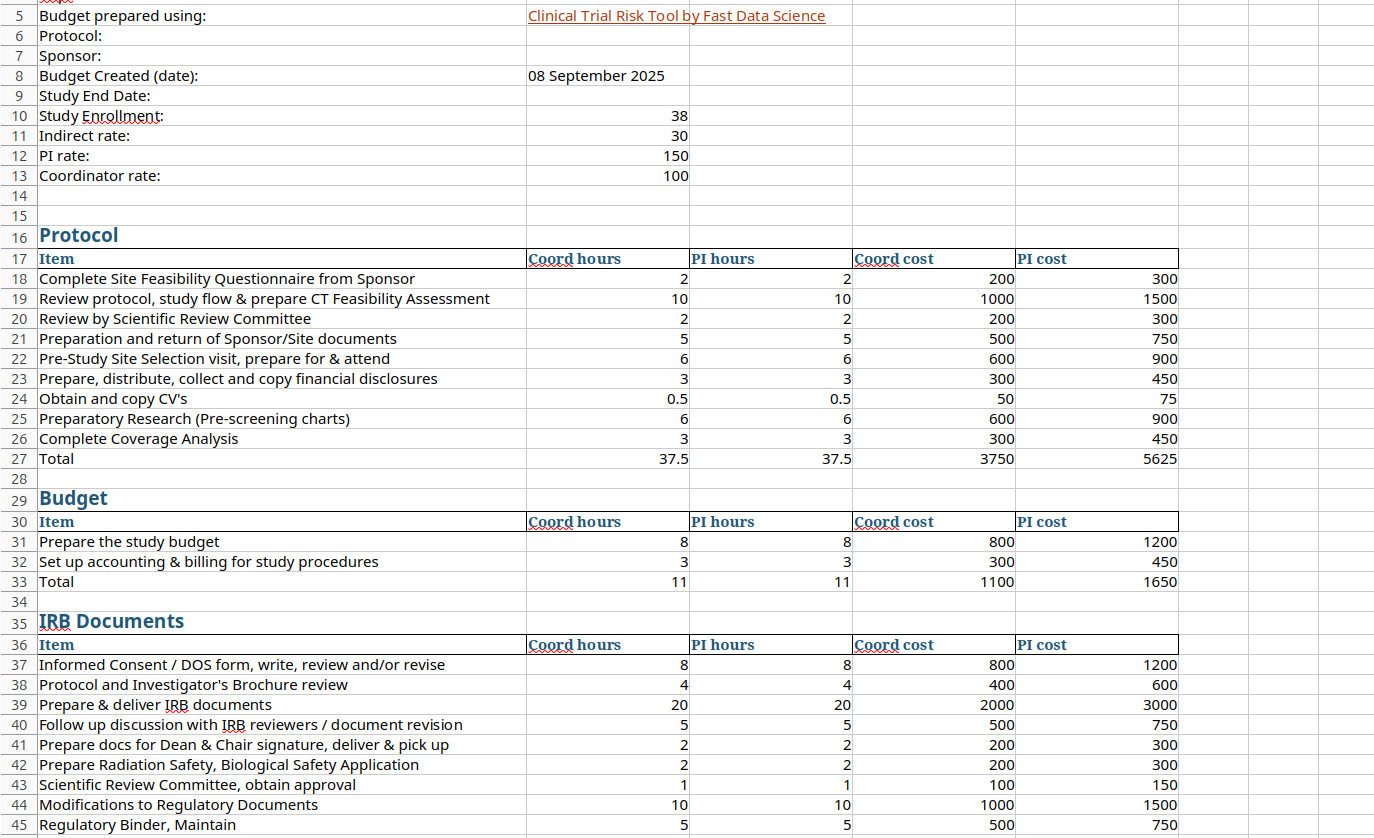
A number of documents are needed to produce a complete and accurate clinical trial budget. The necessary documents typically include: the study protocol (generally the most important document for building a budget) the informed consent form the clinical trial agreement or sponsor contract any laboratory and pharmacy manuals the charge master or schedule of fees the sponsor’s budget. However, sometimes a sponsor will send only the synopsis to a CRO when requesting a quote.

Are you wondering how you can build a detailed clinical trial budget from the protocol, whether for a site, CRO, or sponsor? This may appear an intimidating task. You have to read the protocol carefully, find the schedule of events, calculate how many times each activity occurs during the trial, and slowly create an itemised budget spreadsheet. There are cost items buried in footnotes that you need to look for.

Guest post by Youssef Soliman, medical student at Assiut University and biostatistician In 2025, the outsourcing of clinical trials has become a common strategy for pharmaceutical and biotechnology sponsors. Facing rising R&D costs and complicated studies, sponsors turn to Contract Research Organizations (CROs) and other external partners to manage clinical trials. This practice, known as outsourcing clinical trials, is adopted as a best practice for containing costs and enhancing efficiency in drug development [1].
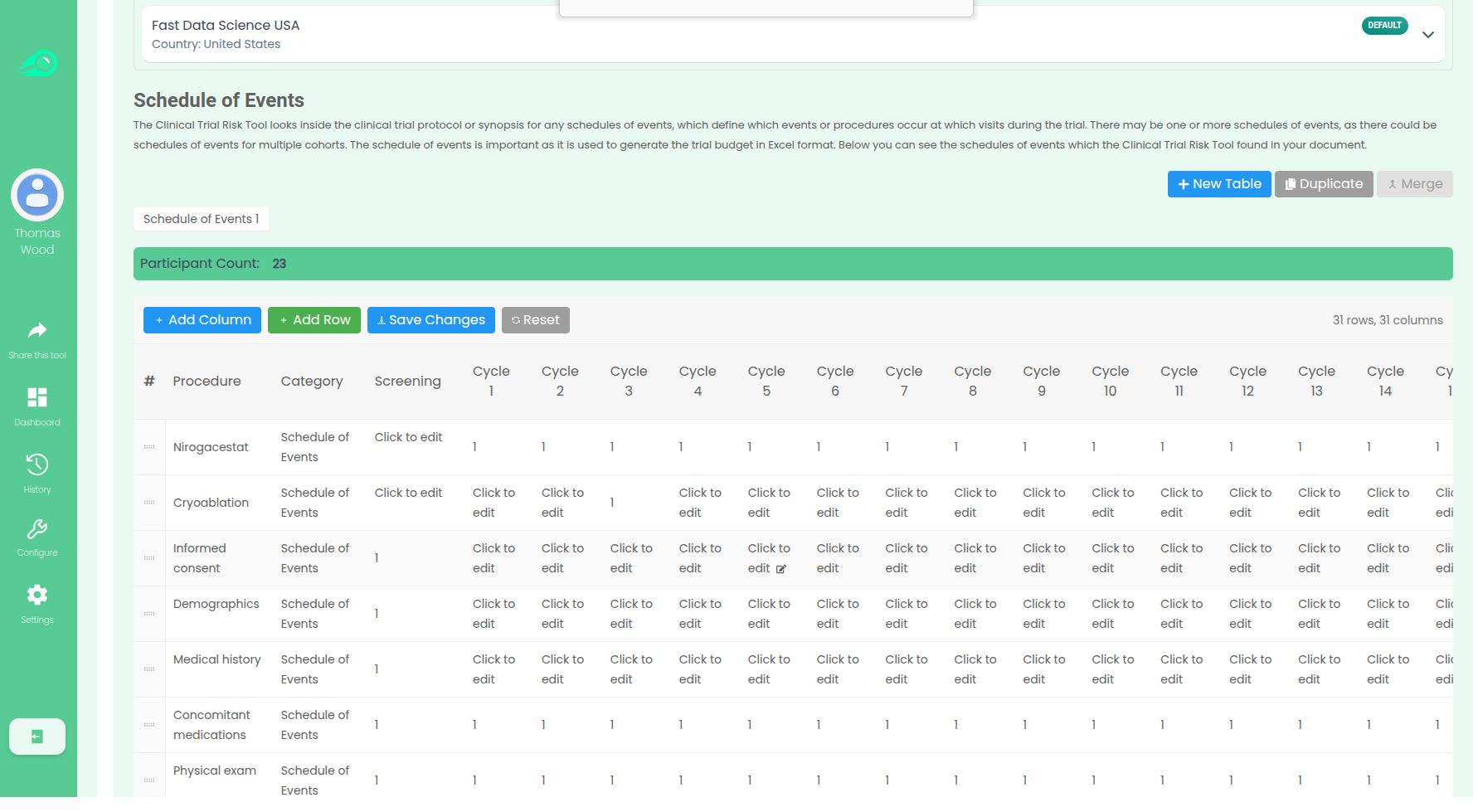
Creating clinical trial budgets from protocols Creating a clinical trial budget is a fiddly and time consuming process. The playbook for running the clinical trial is a document called the protocol. You can find examples of protocols here. The protocol states how many participants will take part in the trial and also what visits and procedures will take place. Above: a protocol. Source: NCT04128579 A clinical trial manager must read the protocol and look for all pieces of information in the protocol that is relevant to the budget, in particular the Schedule of Events (also called Schedule of Assessments or Schedule of Activities), which is a table or series of tables which indicate which procedures and assessments will take place on which the visits.

We have improved the Clinical Trial Risk Tool in the last 6 months, making it more user friendly and taking on board the feedback that we’ve received. We’ve improved the accuracy of the machine learning components too. The tool now outputs its key figures such as risk levels and estimated cost in easily readable cards, so you can see at a glance the key takeaways from your protocol: The risk factors are now organised into collapsible categories, so you can explore them easily without an information overload.

Guest post by Safeer Khan, Lecturer at Department of Pharmaceutical Sciences, Government College University, Lahore, Pakistan Introduction The success of a clinical trial is strongly dependent on the structure and coordination of the teams managing it. Given the high stakes and significant impact of every decision made during the trial, it is essential for each team member to collaborate efficiently in order to meet strict deadlines, comply with regulations, and ensure reliable results.

Guest post by Youssef Soliman, medical student at Assiut University and biostatistician Clinical trial protocols are detailed master-plans of a study – often 100–200 pages long – outlining objectives, design, procedures, eligibility and analysis. Reading them cover-to-cover can be daunting and time-consuming. Yet careful review is essential. Protocols are the “backbone” of good research, ensuring trials are safe for participants and scientifically valid [1]. Fortunately, there are systematic strategies to speed up review and keep it objective.